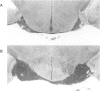
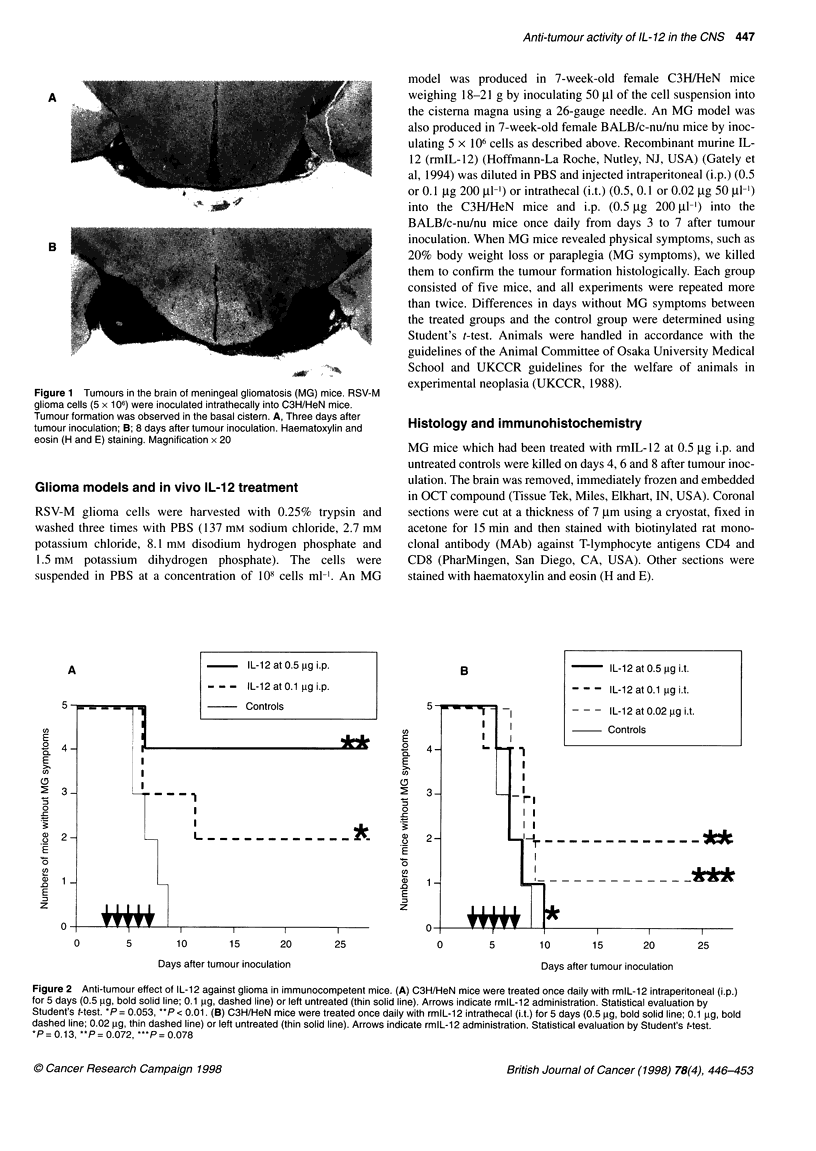
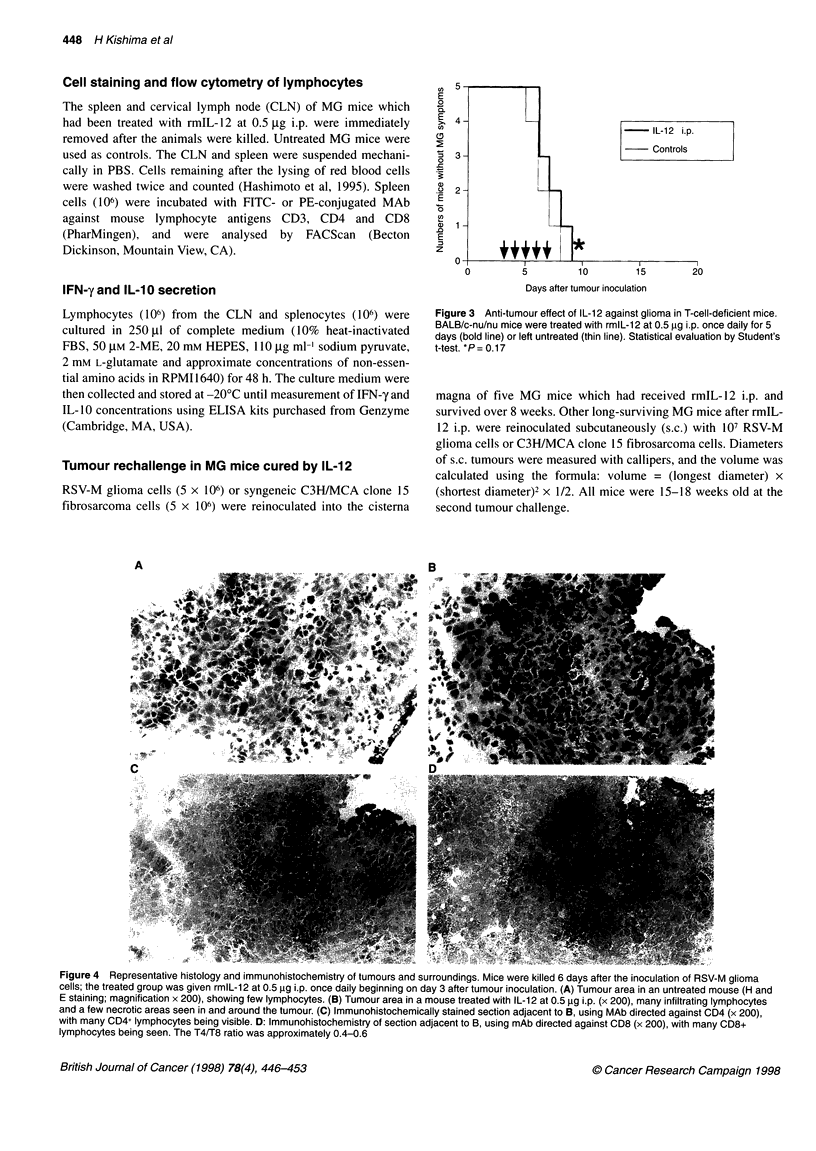
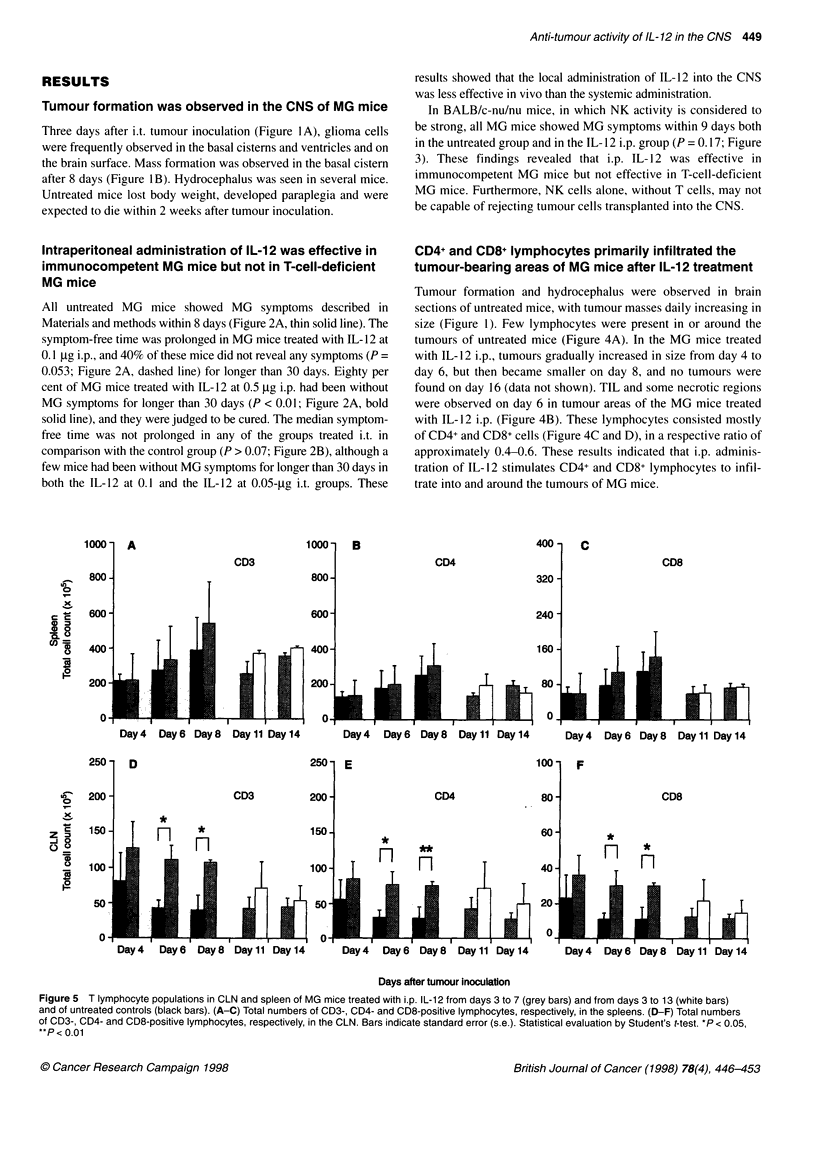
Abstract
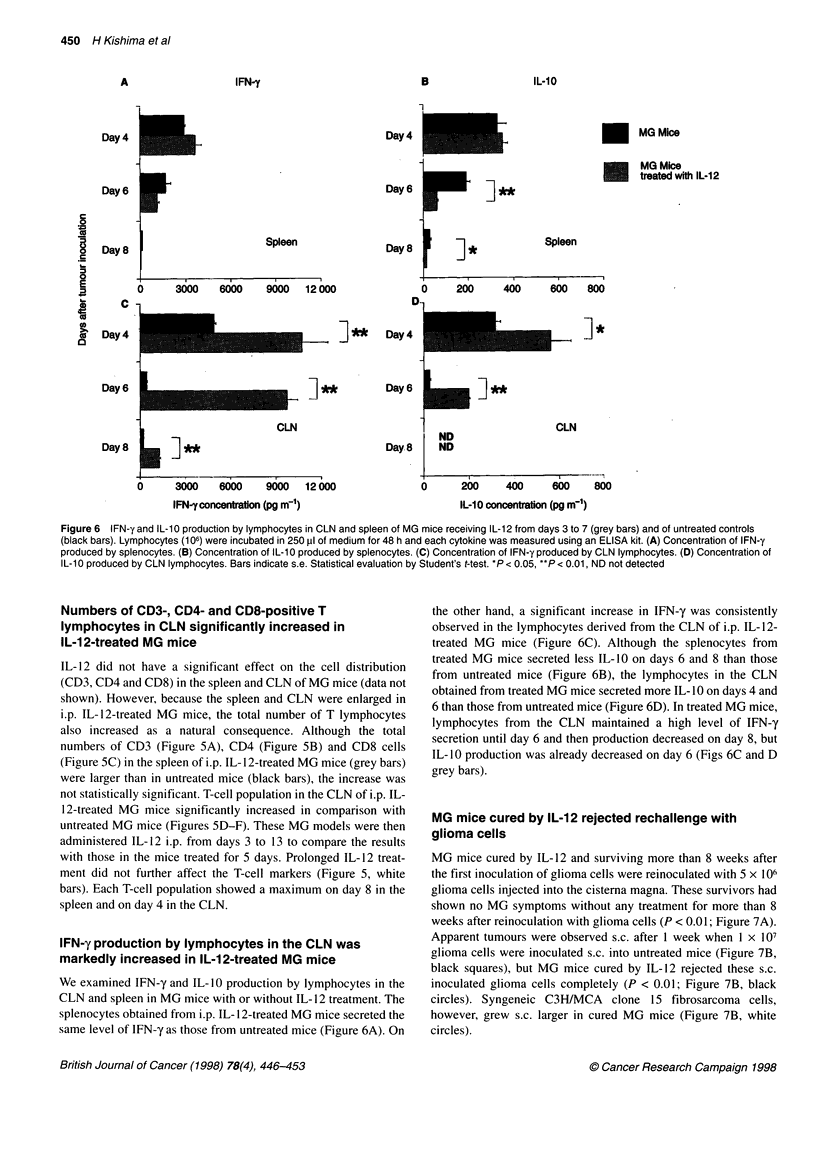
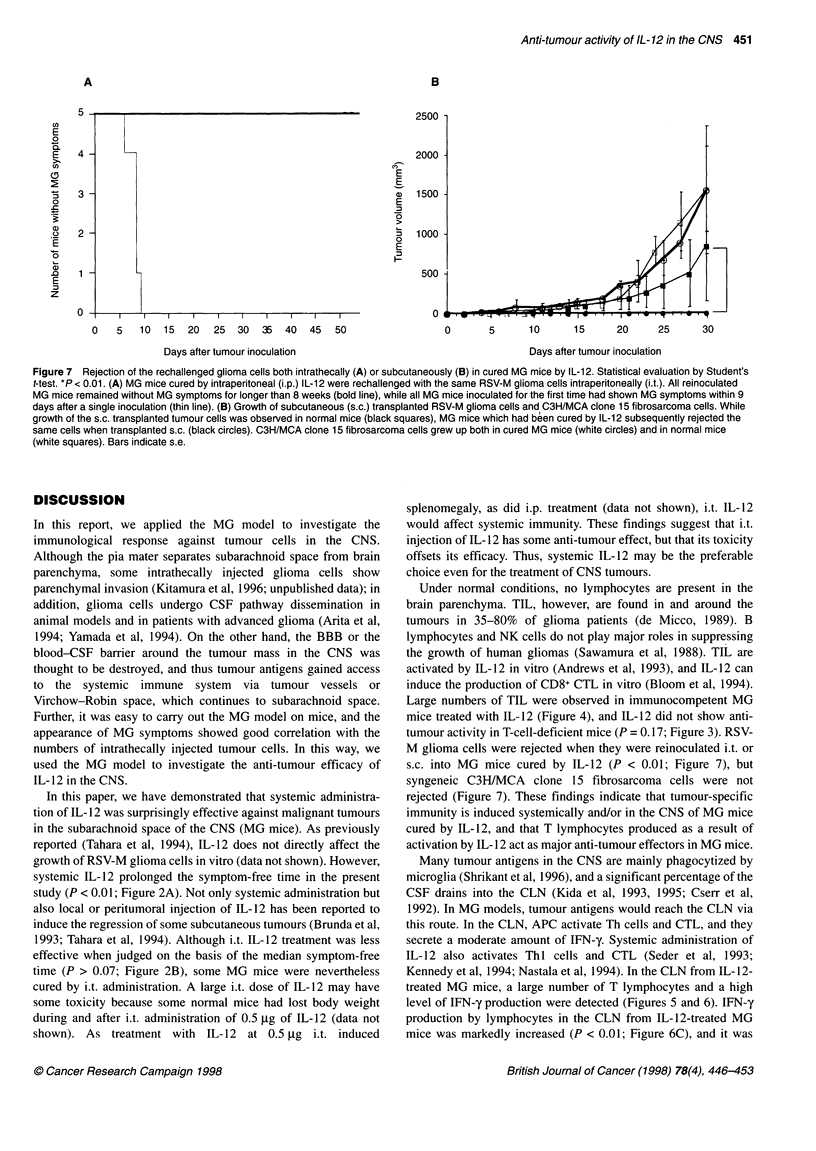
In various systemic cancers, interleukin 12 (IL-12) induces anti-tumour immunity mediated by T lymphocytes and natural killer cells. To determine whether IL-12 has anti-tumour activity against malignant gliomas in the central nervous system (CNS), which is considered to be an immunologically privileged site, we treated mice with meningeal gliomatosis by intraperitoneal (i.p.) or intrathecal (i.t.) administration of recombinant murine IL-12. Although untreated mice revealed symptoms, such as body weight loss or paraplegia as a result of the meningeal gliomatosis within 8 days after tumour inoculation, 80% of the mice treated with IL-12 at 0.5 microg i.p. were cured. Many lymphocytes, mostly CD4+ and CD8+ cells, infiltrated to the tumours of IL-12-treated mice. The numbers of these cells increased in the cervical lymph nodes, into which the cerebrospinal fluid drains, and there they secreted a considerable amount of interferon-gamma. Mice cured by IL-12 rejected subcutaneous or i.t. rechallenge with their original glioma cells, but the same mice were not able to reject other syngeneic tumour cells. These results indicate that the immune system recognizes malignant glioma cells in the subarachnoid space of the CNS and that systemic IL-12 may produce effective anti-tumour activity and long-lasting tumour-specific immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annegers J. F., Schoenberg B. S., Okazaki H., Kurland L. T. Epidemiologic study of primary intracranial neoplasms. Arch Neurol. 1981 Apr;38(4):217–219. doi: 10.1001/archneur.1981.00510040043006. [DOI] [PubMed] [Google Scholar]
- Arita N., Taneda M., Hayakawa T. Leptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcome. Acta Neurochir (Wien) 1994;126(2-4):84–92. doi: 10.1007/BF01476415. [DOI] [PubMed] [Google Scholar]
- Barba D., Saris S. C., Holder C., Rosenberg S. A., Oldfield E. H. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989 Feb;70(2):175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- Bloom E. T., Horvath J. A. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T cell activity. Implications for the age-related decline in CTL. J Immunol. 1994 May 1;152(9):4242–4254. [PubMed] [Google Scholar]
- Brunda M. J., Gately M. K. Antitumor activity of interleukin-12. Clin Immunol Immunopathol. 1994 Jun;71(3):253–255. doi: 10.1006/clin.1994.1081. [DOI] [PubMed] [Google Scholar]
- Brunda M. J. Interleukin-12. J Leukoc Biol. 1994 Feb;55(2):280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesano A., Visonneau S., Santoli D. Treatment of experimental glioblastoma with a human major histocompatibility complex nonrestricted cytotoxic T cell line. Cancer Res. 1995 Jan 1;55(1):96–101. [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., Harling-Berg C. J., Knopf P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992 Oct;2(4):269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Madden K. B., Cheever A. W., Katona I. M., Morris S. C., Gately M. K., Hubbard B. R., Gause W. C., Urban J. F., Jr Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994 May 1;179(5):1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Frei K., Bodmer S., Hofer E. Immune-mediated encephalitis: on the role of antigen-presenting cells in brain tissue. Immunol Rev. 1987 Dec;100:185–201. doi: 10.1111/j.1600-065X.1987.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Frank E., Pulver M., de Tribolet N. Expression of class II major histocompatibility antigens on reactive astrocytes and endothelial cells within the gliosis surrounding metastases and abscesses. J Neuroimmunol. 1986 Jul;12(1):29–36. doi: 10.1016/0165-5728(86)90094-9. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Färkkilä M., Jäskeläinen J., Kallio M., Blomstedt G., Raininko R., Virkkunen P., Paetau A., Sarelin H., Mäntylä M. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994 Jul;70(1):138–141. doi: 10.1038/bjc.1994.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Warrier R. R., Honasoge S., Carvajal D. M., Faherty D. A., Connaughton S. E., Anderson T. D., Sarmiento U., Hubbard B. R., Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994 Jan;6(1):157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- Gerosa F., Paganin C., Peritt D., Paiola F., Scupoli M. T., Aste-Amezaga M., Frank I., Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996 Jun 1;183(6):2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995 May 1;154(9):4333–4340. [PubMed] [Google Scholar]
- Hickey W. F., Vass K., Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992 May;51(3):246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Picha K. S., Shanebeck K. D., Anderson D. M., Grabstein K. H. Interleukin-12 regulates the proliferation of Th1, but not Th2 or Th0, clones. Eur J Immunol. 1994 Oct;24(10):2271–2278. doi: 10.1002/eji.1830241002. [DOI] [PubMed] [Google Scholar]
- Kida S., Pantazis A., Weller R. O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993 Dec;19(6):480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kida S., Weller R. O., Zhang E. T., Phillips M. J., Iannotti F. Anatomical pathways for lymphatic drainage of the brain and their pathological significance. Neuropathol Appl Neurobiol. 1995 Jun;21(3):181–184. doi: 10.1111/j.1365-2990.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Kitamura I., Kochi M., Matsumoto Y., Ueoka R., Kuratsu J., Ushio Y. Intrathecal chemotherapy with 1,3-bis(2-chloroethyl)-1-nitrosourea encapsulated into hybrid liposomes for meningeal gliomatosis: an experimental study. Cancer Res. 1996 Sep 1;56(17):3986–3992. [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanishi T., Ikuta F., Yamamoto T. Brain tumors induced by Rous sarcoma virus, Schmidt-Ruppin strain. 3. Morphology of brain tumors induced in adult mice. J Natl Cancer Inst. 1973 Jan;50(1):95–109. doi: 10.1093/jnci/50.1.95. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Liu W., Brosnan C. F., Dickson D. W. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994 Dec;12(4):309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- Lillehei K. O., Mitchell D. H., Johnson S. D., McCleary E. L., Kruse C. A. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991 Jan;28(1):16–23. doi: 10.1097/00006123-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Marshall J. D., Secrist H., DeKruyff R. H., Wolf S. F., Umetsu D. T. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995 Jul 1;155(1):111–117. [PubMed] [Google Scholar]
- Martinotti A., Stoppacciaro A., Vagliani M., Melani C., Spreafico F., Wysocka M., Parmiani G., Trinchieri G., Colombo M. P. CD4 T cells inhibit in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with interleukin-12 genes. Eur J Immunol. 1995 Jan;25(1):137–146. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ohmori K., Fujiwara M. Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology. 1992 Jun;76(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Mu J., Zou J. P., Yamamoto N., Tsutsui T., Tai X. G., Kobayashi M., Herrmann S., Fujiwara H., Hamaoka T. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res. 1995 Oct 1;55(19):4404–4408. [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Nazzaro J. M., Neuwelt E. A. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 1990 Sep;73(3):331–344. doi: 10.3171/jns.1990.73.3.0331. [DOI] [PubMed] [Google Scholar]
- Nitta T., Sato K., Yagita H., Okumura K., Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990 Feb 17;335(8686):368–371. doi: 10.1016/0140-6736(90)90205-j. [DOI] [PubMed] [Google Scholar]
- Ohno K., Suzumura A., Sawada M., Marunouchi T. Production of granulocyte/macrophage colony-stimulating factor by cultured astrocytes. Biochem Biophys Res Commun. 1990 Jun 15;169(2):719–724. doi: 10.1016/0006-291x(90)90390-9. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Sawamura Y., Abe H., Aida T., Hosokawa M., Kobayashi H. Isolation and in vitro growth of glioma-infiltrating lymphocytes, and an analysis of their surface phenotypes. J Neurosurg. 1988 Nov;69(5):745–750. doi: 10.3171/jns.1988.69.5.0745. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Chua A. O., Wolitzky A. G., Quinn P. M., Dwyer C. M., McComas W., Familletti P. C., Gately M. K., Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992 Jun 1;148(11):3433–3440. [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Okamoto Y., Miyao Y., Yamada M., Ushio Y., Hayakawa T., Ikeda H., Mogami H. Adoptive immunotherapy of human meningeal gliomatosis and carcinomatosis with LAK cells and recombinant interleukin-2. J Neurosurg. 1987 Apr;66(4):519–521. doi: 10.3171/jns.1987.66.4.0519. [DOI] [PubMed] [Google Scholar]
- Shrikant P., Benveniste E. N. The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol. 1996 Sep 1;157(5):1819–1822. [PubMed] [Google Scholar]
- Stern A. S., Podlaski F. J., Hulmes J. D., Pan Y. C., Quinn P. M., Wolitzky A. G., Familletti P. C., Stremlo D. L., Truitt T., Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H., Lotze M. T. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995 Mar;2(2):96–106. [PubMed] [Google Scholar]
- Tahara H., Zeh H. J., 3rd, Storkus W. J., Pappo I., Watkins S. C., Gubler U., Wolf S. F., Robbins P. D., Lotze M. T. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994 Jan 1;54(1):182–189. [PubMed] [Google Scholar]
- Tamura K., Shimizu K., Yamada M., Okamoto Y., Matsui Y., Park K. C., Mabuchi E., Moriuchi S., Mogami H. Expression of major histocompatibility complex on human medulloblastoma cells with neuronal differentiation. Cancer Res. 1989 Oct 1;49(19):5380–5384. [PubMed] [Google Scholar]
- Wolf S. F., Temple P. A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R. M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Shimizu K., Miyao Y., Hayakawa T., Nakajima K., Nakahira K., Nakagawa H., Mikoshiba K., Ikenaka K. Migration of genetically labeled glioma cells after implantation into murine brain. J Neurosci Res. 1994 Jul 1;38(4):415–423. doi: 10.1002/jnr.490380407. [DOI] [PubMed] [Google Scholar]
- Yoshida J., Kajita Y., Wakabayashi T., Sugita K. Long-term follow-up results of 175 patients with malignant glioma: importance of radical tumour resection and postoperative adjuvant therapy with interferon, ACNU and radiation. Acta Neurochir (Wien) 1994;127(1-2):55–59. doi: 10.1007/BF01808547. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Robbins P. D., Storkus W. J., Clarke M. R., Maeurer M. J., Campbell R. L., Davis C. G., Tahara H., Schreiber R. D., Lotze M. T. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol. 1996 Jun;26(6):1335–1341. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- de Micco C. Immunology of central nervous system tumors. J Neuroimmunol. 1989 Dec;25(2-3):93–108. doi: 10.1016/0165-5728(89)90127-6. [DOI] [PubMed] [Google Scholar]