Abstract
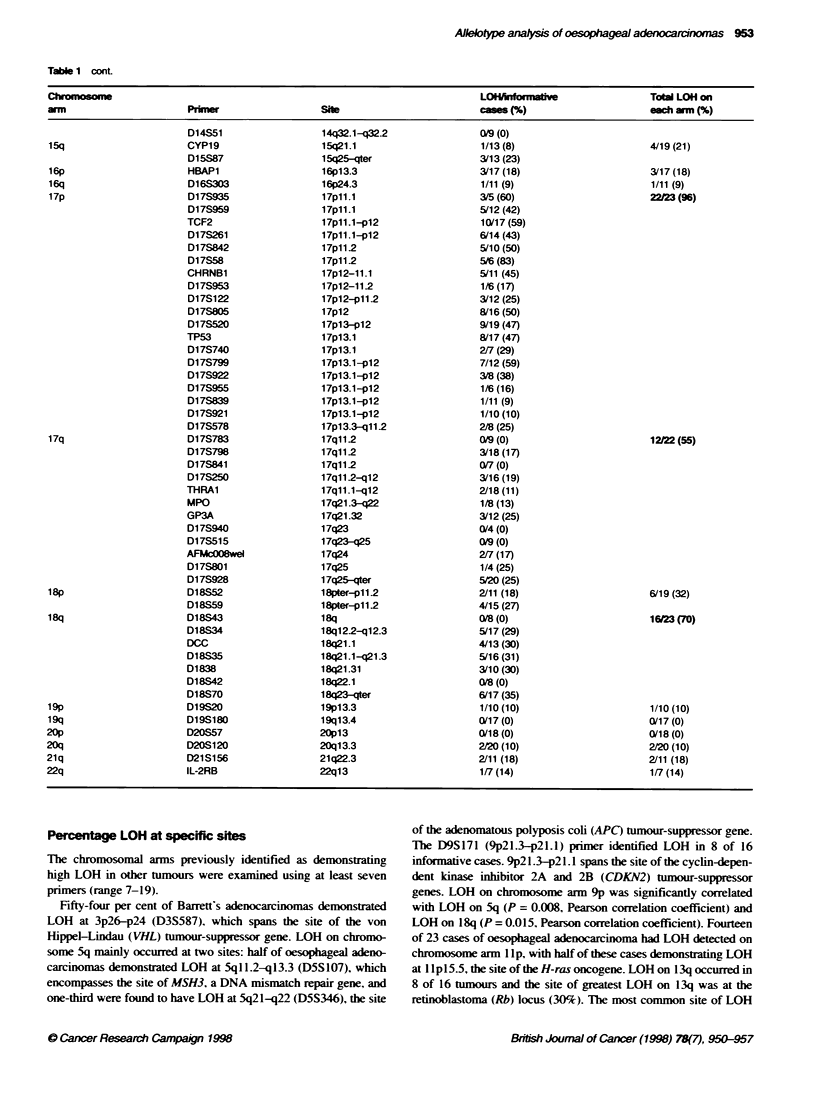
Deletions of tumour-suppressor genes can be detected by loss of heterozygosity (LOH) studies, which were performed on 23 cases of adenocarcinoma of the oesophagus, using 120 microsatellite primers covering all non-acrocentric autosomal chromosome arms. The chromosomal arms most frequently demonstrating LOH were 3p (64% of tumours), 5q (45%), 9p (52%), 11p (61%), 13q (50%), 17p (96%), 17q (55%) and 18q (70%). LOH on 3p, 9p, 13q, 17p and 18q occurred mainly within the loci of the VHL, CDKN2, Rb, TP53 and DCC tumour-suppressor genes respectively. LOH on 5q occurred at the sites of the MSH3 mismatch repair gene and the APC tumour-suppressor gene. 11p15.5 and 17q25-qter represented areas of greatest LOH on chromosomes 11p and 17q, and are putative sites of novel tumour-suppressor genes. LOH on 9p was significantly associated with LOH on 5q, and tumours demonstrating LOH at both the CDKN2 (9p21) and MSH3 (5q11-q12) genes had a significantly higher fractional allele loss than those retaining heterozygosity at these sites. Six of nine carcinomas displaying microsatellite alterations also demonstrated LOH at CDKN2, which may be associated with widespread genomic instability. Overall, there are nine sites of LOH associated with oesophageal adenocarcinoma.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gleeson C. M., Sloan J. M., McGuigan J. A., Ritchie A. J., Russell S. E. Base transitions at CpG dinucleotides in the p53 gene are common in esophageal adenocarcinoma. Cancer Res. 1995 Aug 1;55(15):3406–3411. [PubMed] [Google Scholar]
- Hamelin R., Fléjou J. F., Muzeau F., Potet F., Laurent-Puig P., Fékété F., Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994 Oct;107(4):1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Hammoud Z. T., Kaleem Z., Cooper J. D., Sundaresan R. S., Patterson G. A., Goodfellow P. J. Allelotype analysis of esophageal adenocarcinomas: evidence for the involvement of sequences on the long arm of chromosome 4. Cancer Res. 1996 Oct 1;56(19):4499–4502. [PubMed] [Google Scholar]
- Kalikin L. M., Frank T. S., Svoboda-Newman S. M., Wetzel J. C., Cooney K. A., Petty E. M. A region of interstitial 17q25 allelic loss in ovarian tumors coincides with a defined region of loss in breast tumors. Oncogene. 1997 Apr 24;14(16):1991–1994. doi: 10.1038/sj.onc.1201013. [DOI] [PubMed] [Google Scholar]
- Keller G., Rotter M., Vogelsang H., Bischoff P., Becker K. F., Mueller J., Brauch H., Siewert J. R., Höfler H. Microsatellite instability in adenocarcinomas of the upper gastrointestinal tract. Relation to clinicopathological data and family history. Am J Pathol. 1995 Sep;147(3):593–600. [PMC free article] [PubMed] [Google Scholar]
- Miros M., Kerlin P., Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett's oesophagus. Gut. 1991 Dec;32(12):1441–1446. doi: 10.1136/gut.32.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara S., Maesawa C., Tamura G., Satodate R. Frequent microsatellite alterations on chromosome 3p in esophageal squamous cell carcinoma. Cancer Res. 1995 Feb 15;55(4):891–894. [PubMed] [Google Scholar]
- Ogasawara S., Tamura G., Maesawa C., Suzuki Y., Ishida K., Satoh N., Uesugi N., Saito K., Satodate R. Common deleted region on the long arm of chromosome 5 in esophageal carcinoma. Gastroenterology. 1996 Jan;110(1):52–57. doi: 10.1053/gast.1996.v110.pm8536888. [DOI] [PubMed] [Google Scholar]
- Ohta M., Inoue H., Cotticelli M. G., Kastury K., Baffa R., Palazzo J., Siprashvili Z., Mori M., McCue P., Druck T. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996 Feb 23;84(4):587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Porschen R., Bevers G., Remy U., Schauseil S., Borchard F. Influence of preoperative radiotherapy on DNA ploidy in squamous cell carcinomas of the oesophagus. Gut. 1993 Aug;34(8):1086–1090. doi: 10.1136/gut.34.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. M., Papadopoulos N., Kinzler K. W., Smolinski K. N., Meltzer S. J. APC gene mutations in the mutation cluster region are rare in esophageal cancers. Gastroenterology. 1994 Dec;107(6):1759–1763. doi: 10.1016/0016-5085(94)90818-4. [DOI] [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Roth G., Surawicz C. M., Van Belle G., Lewin K., Weinstein W. M., Antonioli D. A., Goldman H. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol. 1988 Feb;19(2):166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- Shibagaki I., Shimada Y., Wagata T., Ikenaga M., Imamura M., Ishizaki K. Allelotype analysis of esophageal squamous cell carcinoma. Cancer Res. 1994 Jun 1;54(11):2996–3000. [PubMed] [Google Scholar]
- Sozzi G., Veronese M. L., Negrini M., Baffa R., Cotticelli M. G., Inoue H., Tornielli S., Pilotti S., De Gregorio L., Pastorino U. The FHIT gene 3p14.2 is abnormal in lung cancer. Cell. 1996 Apr 5;85(1):17–26. doi: 10.1016/s0092-8674(00)81078-8. [DOI] [PubMed] [Google Scholar]
- Spechler S. J., Robbins A. H., Rubins H. B., Vincent M. E., Heeren T., Doos W. G., Colton T., Schimmel E. M. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984 Oct;87(4):927–933. [PubMed] [Google Scholar]
- Spechler S. J., Zeroogian J. M., Antonioli D. A., Wang H. H., Goyal R. K. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994 Dec 3;344(8936):1533–1536. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- Swift A., Risk J. M., Kingsnorth A. N., Wright T. A., Myskow M., Field J. K. Frequent loss of heterozygosity on chromosome 17 at 17q11.2-q12 in Barrett's adenocarcinoma. Br J Cancer. 1995 May;71(5):995–998. doi: 10.1038/bjc.1995.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Waber P. G., Lee N. K., Nisen P. D. Frequent allelic loss at chromosome arm 3p is distinct from genetic alterations of the Von-Hippel Lindau tumor suppressor gene in head and neck cancer. Oncogene. 1996 Jan 18;12(2):365–369. [PubMed] [Google Scholar]
- Wagata T., Ishizaki K., Imamura M., Shimada Y., Ikenaga M., Tobe T. Deletion of 17p and amplification of the int-2 gene in esophageal carcinomas. Cancer Res. 1991 Apr 15;51(8):2113–2117. [PubMed] [Google Scholar]
- Walsh T. N., Dorman T., Droogan O., Curran B., Hourihane D. O., Leader M., Hennessy T. P. DNA ploidy in squamous oesophageal carcinoma. Surg Oncol. 1992 Feb;1(1):37–42. doi: 10.1016/0960-7404(92)90054-o. [DOI] [PubMed] [Google Scholar]
- Williamson W. A., Ellis F. H., Jr, Gibb S. P., Shahian D. M., Aretz H. T., Heatley G. J., Watkins E., Jr Barrett's esophagus. Prevalence and incidence of adenocarcinoma. Arch Intern Med. 1991 Nov;151(11):2212–2216. doi: 10.1001/archinte.151.11.2212. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Toguchida J., Yamamuro T., Kotoura Y., Takada N., Kawaguchi N., Kaneko Y., Nakamura Y., Sasaki M. S., Ishizaki K. Allelotype analysis in osteosarcomas: frequent allele loss on 3q, 13q, 17p, and 18q. Cancer Res. 1992 May 1;52(9):2419–2423. [PubMed] [Google Scholar]
- Zhuang Z., Emmert-Buck M. R., Roth M. J., Gnarra J., Linehan W. M., Liotta L. A., Lubensky I. A. von Hippel-Lindau disease gene deletion detected in microdissected sporadic human colon carcinoma specimens. Hum Pathol. 1996 Feb;27(2):152–156. doi: 10.1016/s0046-8177(96)90368-8. [DOI] [PubMed] [Google Scholar]
- van den Berg A., Hulsbeek M. F., de Jong D., Kok K., Veldhuis P. M., Roche J., Buys C. H. Major role for a 3p21 region and lack of involvement of the t(3;8) breakpoint region in the development of renal cell carcinoma suggested by loss of heterozygosity analysis. Genes Chromosomes Cancer. 1996 Jan;15(1):64–72. doi: 10.1002/(SICI)1098-2264(199601)15:1<64::AID-GCC9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]



