Abstract
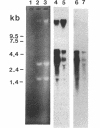
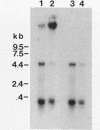
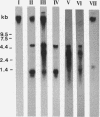
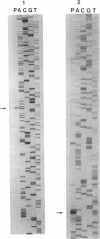
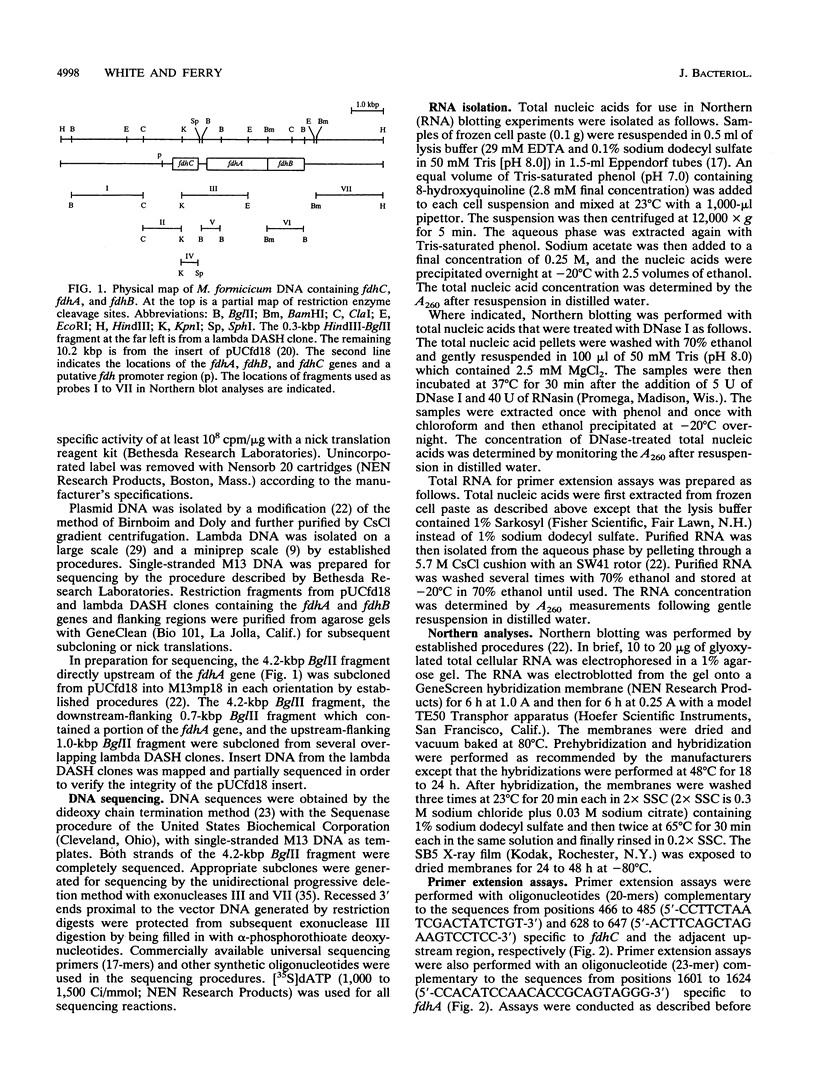
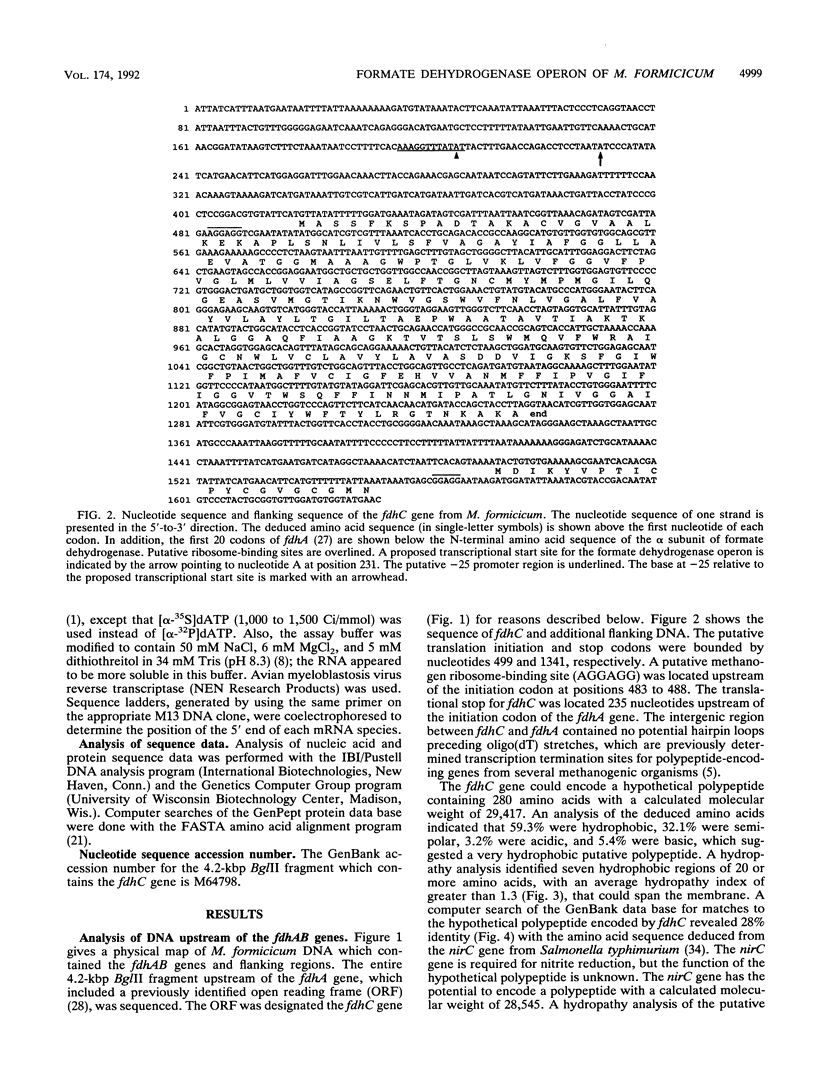
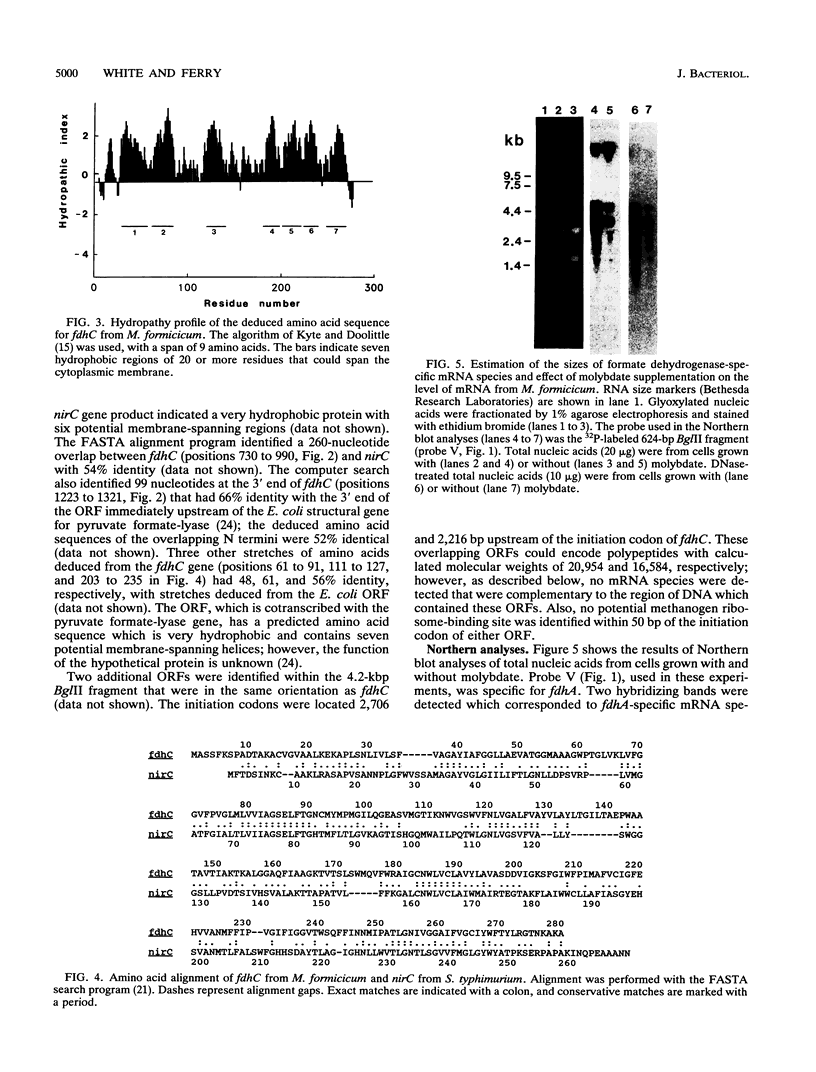
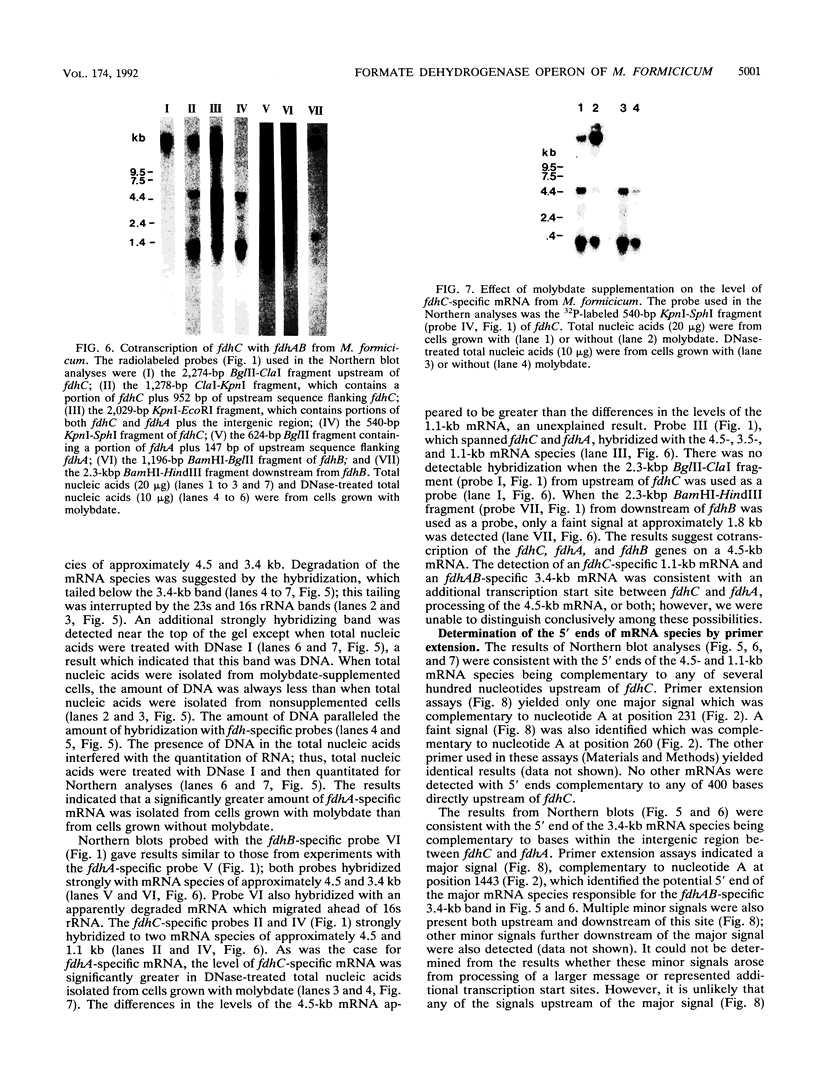
The overlapping fdhA and fdhB genes of Methanobacterium formicicum, which encode the alpha and beta subunits, respectively, of formate dehydrogenase, were cotranscribed as part of an approximately 4.5-kb transcript. An additional gene (fdhC) upstream of fdhA was cotranscribed with fdhA and fdhB. The deduced amino acid sequence suggested that fdhC has the potential to encode a hydrophobic polypeptide with a calculated molecular weight of 29,417. A hydropathy plot of the hypothetical polypeptide indicated several potential membrane-spanning regions. The putative fdhC gene product had 28% identity with the deduced amino acid sequence of the nirC gene from Salmonella typhimurium. Northern (RNA) blot analyses and primer extension assays located a transcription start site 268 bp upstream of the initiation codon of fdhC. A sequence identical to the consensus promoter sequence for methanogenic organisms was situated between -35 and -25 bp from the proposed transcription start site. In addition to the 4.5-kb transcript, Northern blot analyses detected a 1.1-kb transcript with an fdhC-specific probe and a 3.4-kb transcript with either an fdhA- or fdhB-specific probe. The levels of all three transcripts were significantly greater in cells grown in media supplemented with molybdate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Whitaker R. A., Krogmann D. W., Curtis S. E. Isolation and sequence of the gene for ferredoxin I from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1986 Dec;168(3):1265–1271. doi: 10.1128/jb.168.3.1265-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birkmann A., Zinoni F., Sawers G., Böck A. Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli. Arch Microbiol. 1987 Jun;148(1):44–51. doi: 10.1007/BF00429646. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Daniels C. J., Reeve J. N. Gene structure, organization, and expression in archaebacteria. Crit Rev Microbiol. 1989;16(4):287–338. doi: 10.3109/10408418909105479. [DOI] [PubMed] [Google Scholar]
- Cotter P. A., Gunsalus R. P. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3817–3823. doi: 10.1128/jb.171.7.3817-3823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G., Thomm M., Brüdigam B., Gohl H. P., Hausner W. An archaebacterial cell-free transcription system. The expression of tRNA genes from Methanococcus vannielii is mediated by a transcription factor. Nucleic Acids Res. 1990 Mar 25;18(6):1361–1367. doi: 10.1093/nar/18.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberger D. Minipreps of DNA from bacteriophage lambda. Nucleic Acids Res. 1987 Aug 25;15(16):6737–6737. doi: 10.1093/nar/15.16.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner W., Frey G., Thomm M. Control regions of an archaeal gene. A TATA box and an initiator element promote cell-free transcription of the tRNA(Val) gene of Methanococcus vannielii. J Mol Biol. 1991 Dec 5;222(3):495–508. doi: 10.1016/0022-2836(91)90492-o. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Molybdenum effector of fumarate reductase repression and nitrate reductase induction in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3720–3725. doi: 10.1128/jb.169.8.3720-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Bastian N. R., Schauer N. L., Ferry J. G., Rajagopalan K. V. Identification of molybdopterin guanine dinucleotide in formate dehydrogenase from Methanobacterium formicicum. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):213–216. doi: 10.1016/0378-1097(91)90554-n. [DOI] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. Nitrate- and molybdenum-independent signal transduction mutations in narX that alter regulation of anaerobic respiratory genes in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7049–7056. doi: 10.1128/jb.172.12.7049-7056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Luque F., Pau R. N. Transcriptional regulation by metals of structural genes for Azotobacter vinelandii nitrogenases. Mol Gen Genet. 1991 Jul;227(3):481–487. doi: 10.1007/BF00273941. [DOI] [PubMed] [Google Scholar]
- May H. D., Patel P. S., Ferry J. G. Effect of molybdenum and tungsten on synthesis and composition of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1988 Aug;170(8):3384–3389. doi: 10.1128/jb.170.8.3384-3389.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Scott D. J., Amy N. K. Molybdenum-sensitive transcriptional regulation of the chlD locus of Escherichia coli. J Bacteriol. 1987 May;169(5):1853–1860. doi: 10.1128/jb.169.5.1853-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. S., Ferry J. G. Characterization of the upstream region of the formate dehydrogenase operon of Methanobacterium formicicum. J Bacteriol. 1988 Aug;170(8):3390–3395. doi: 10.1128/jb.170.8.3390-3395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Böck A. Novel transcriptional control of the pyruvate formate-lyase gene: upstream regulatory sequences and multiple promoters regulate anaerobic expression. J Bacteriol. 1989 May;171(5):2485–2498. doi: 10.1128/jb.171.5.2485-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Composition of the coenzyme F420-dependent formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1986 Feb;165(2):405–411. doi: 10.1128/jb.165.2.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G., Honek J. F., Orme-Johnson W. H., Walsh C. Mechanistic studies of the coenzyme F420 reducing formate dehydrogenase from Methanobacterium formicicum. Biochemistry. 1986 Nov 4;25(22):7163–7168. doi: 10.1021/bi00370a059. [DOI] [PubMed] [Google Scholar]
- Shuber A. P., Orr E. C., Recny M. A., Schendel P. F., May H. D., Schauer N. L., Ferry J. G. Cloning, expression, and nucleotide sequence of the formate dehydrogenase genes from Methanobacterium formicicum. J Biol Chem. 1986 Oct 5;261(28):12942–12947. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomm M., Wich G., Brown J. W., Frey G., Sherf B. A., Beckler G. S. An archaebacterial promoter sequence assigned by RNA polymerase binding experiments. Can J Microbiol. 1989 Jan;35(1):30–35. doi: 10.1139/m89-005. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Siegel L. M., Kredich N. M. High-level expression of Escherichia coli NADPH-sulfite reductase: requirement for a cloned cysG plasmid to overcome limiting siroheme cofactor. J Bacteriol. 1991 Jan;173(1):325–333. doi: 10.1128/jb.173.1.325-333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]