Abstract
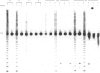
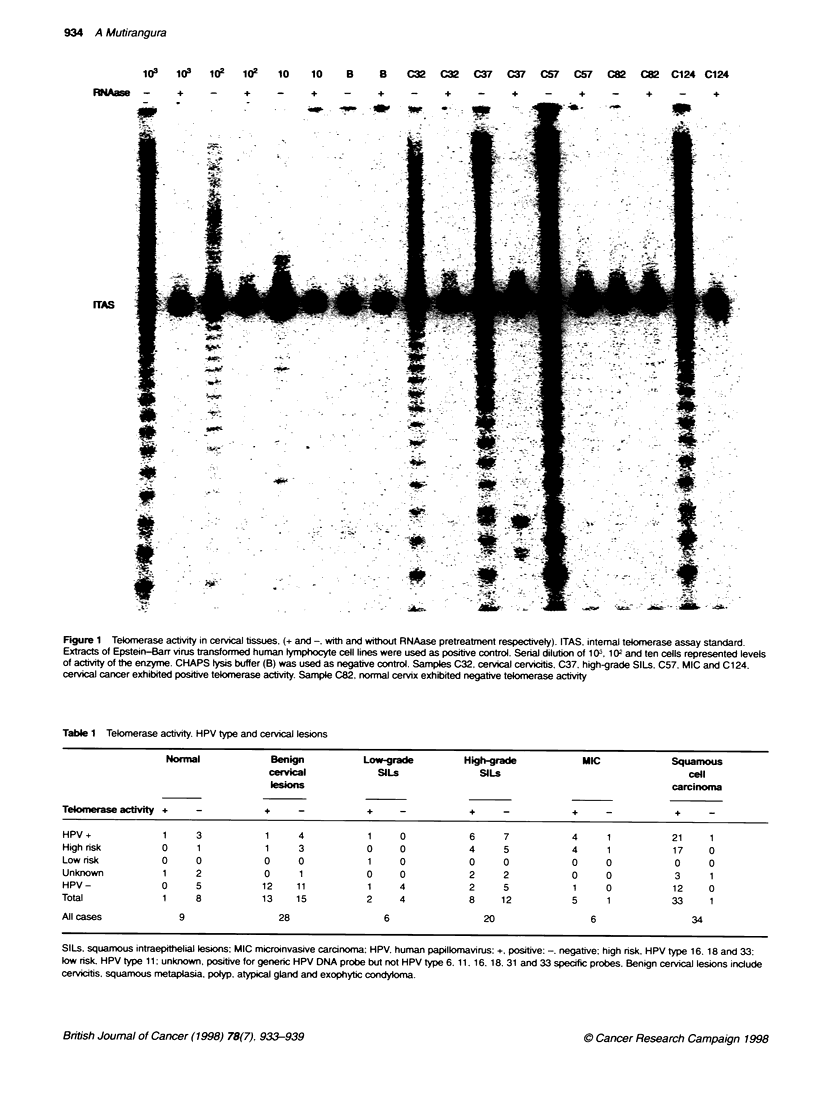
The purpose of this study was to define a correlation between telomerase activity and human papillomavirus (HPV) in normal control tissue and in benign, premalignant and malignant cervical lesions. Telomerase activity was detectable in 33 out of 34 cases of squamous-cell carcinoma, five out of six cases of microinvasive carcinoma, 8 out of 20 cases and two out of six cases of high- and low-grade squamous intraepithelial lesions (SILs) respectively. The higher frequency of positive telomerase in invasive carcinoma compared with SILs was observed in both HPV-associated and non-associated groups. Whereas 92.6% of HPV-positive and 100% of HPV-negative invasive lesions expressed telomerase, only 50% of HPV-positive and 25% of HPV-negative SILs did. Interestingly, telomerase activity was also detectable in 13 out of 28 cases of benign lesions regardless of the presence of HPV. In conclusion, there may be two roles of telomerase in the cervix. The first one would present in benign lesions; the second is associated with cancer development and activated during the late stage of multistep carcinogenesis in both HPV-positive and -negative groups.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Manos M. M., Muñoz N., Sherman M., Jansen A. M., Peto J., Schiffman M. H., Moreno V., Kurman R., Shah K. V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Burger M. P., Hollema H., Pieters W. J., Schröder F. P., Quint W. G. Epidemiological evidence of cervical intraepithelial neoplasia without the presence of human papillomavirus. Br J Cancer. 1996 Mar;73(6):831–836. doi: 10.1038/bjc.1996.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo J. A., Popescu N. C., Alvarez L., Woodworth C. D. Cellular and molecular alterations in human epithelial cells transformed by recombinant human papillomavirus DNA. Crit Rev Oncog. 1993;4(4):337–360. [PubMed] [Google Scholar]
- Engelhardt M., Kumar R., Albanell J., Pettengell R., Han W., Moore M. A. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood. 1997 Jul 1;90(1):182–193. [PubMed] [Google Scholar]
- Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Wu F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 1997 Jul 1;25(13):2595–2597. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhutz A. J., Foster S. A., McDougall J. K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996 Mar 7;380(6569):79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Kyo S., Takakura M., Ishikawa H., Sasagawa T., Satake S., Tateno M., Inoue M. Application of telomerase assay for the screening of cervical lesions. Cancer Res. 1997 May 15;57(10):1863–1867. [PubMed] [Google Scholar]
- Mutirangura A., Supiyaphun P., Trirekapan S., Sriuranpong V., Sakuntabhai A., Yenrudi S., Voravud N. Telomerase activity in oral leukoplakia and head and neck squamous cell carcinoma. Cancer Res. 1996 Aug 1;56(15):3530–3533. [PubMed] [Google Scholar]
- Pao C. C., Tseng C. J., Lin C. Y., Yang F. P., Hor J. J., Yao D. S., Hsueh S. Differential expression of telomerase activity in human cervical cancer and cervical intraepithelial neoplasia lesions. J Clin Oncol. 1997 May;15(5):1932–1937. doi: 10.1200/JCO.1997.15.5.1932. [DOI] [PubMed] [Google Scholar]
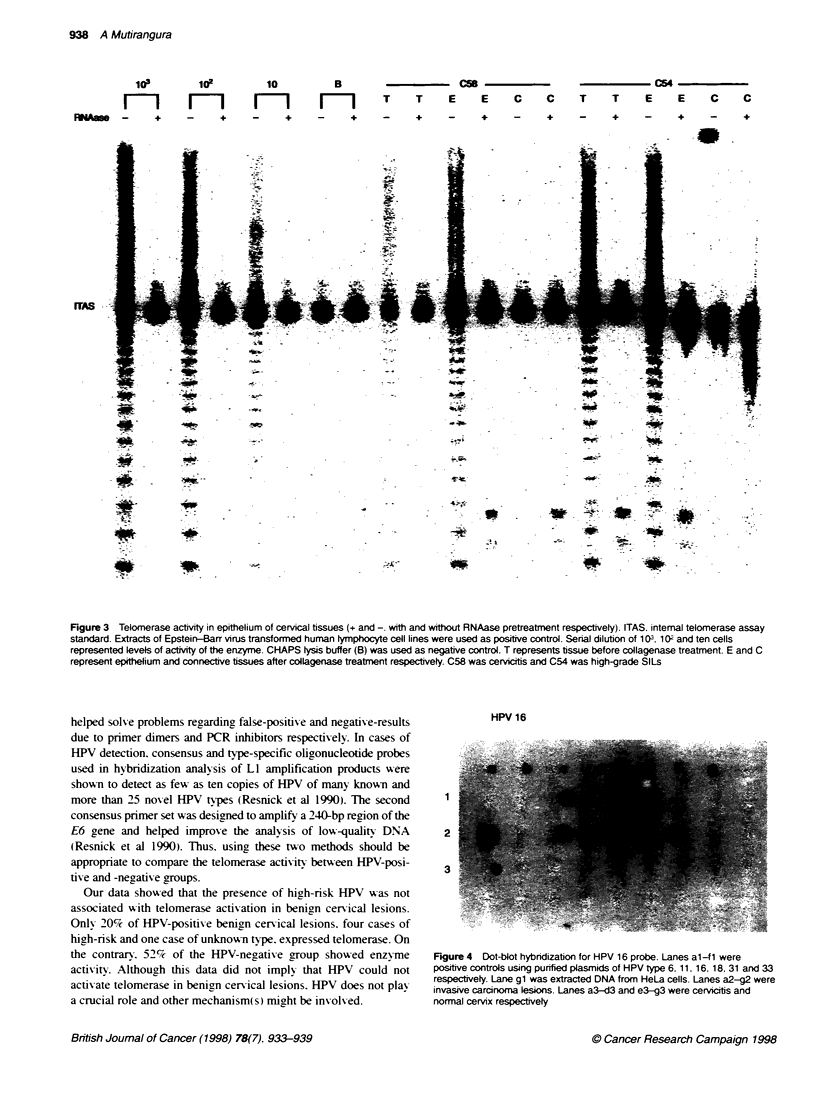
- Resnick R. M., Cornelissen M. T., Wright D. K., Eichinger G. H., Fox H. S., ter Schegget J., Manos M. M. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990 Sep 19;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H., Bauer H. M., Hoover R. N., Glass A. G., Cadell D. M., Rush B. B., Scott D. R., Sherman M. E., Kurman R. J., Wacholder S. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Gazdar A. F. Telomerase in the early detection of cancer. J Clin Pathol. 1997 Feb;50(2):106–109. doi: 10.1136/jcp.50.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen R. D., Walboomers J. M., Meijer C. J., van der Raaij-Helmer E. M., Parker J. N., Chow L. T., Broker T. R., Snijders P. J. Transition of human papillomavirus type 16 and 18 transfected human foreskin keratinocytes towards immortality: activation of telomerase and allele losses at 3p, 10p, 11q and/or 18q. Oncogene. 1996 Sep 19;13(6):1249–1257. [PubMed] [Google Scholar]
- Stöppler H., Hartmann D. P., Sherman L., Schlegel R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem. 1997 May 16;272(20):13332–13337. doi: 10.1074/jbc.272.20.13332. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Ramirez R. D., Ogoshi M., Chaffins M., Piatyszek M. A., Shay J. W. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996 Apr;106(4):759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA. 1989 Aug 18;262(7):931–934. [PubMed] [Google Scholar]
- Wright W. E., Shay J. W., Piatyszek M. A. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995 Sep 25;23(18):3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Kunimura C., Kikuchi K., Tahara H., Ohji H., Yamamoto H., Ide T., Utakoji T. Telomerase activity in normal human epithelial cells. Oncogene. 1996 Jul 18;13(2):433–439. [PubMed] [Google Scholar]
- Zheng P. S., Iwasaka T., Yokoyama M., Nakao Y., Pater A., Sugimori H. Telomerase activation in in vitro and in vivo cervical carcinogenesis. Gynecol Oncol. 1997 Aug;66(2):222–226. doi: 10.1006/gyno.1997.4757. [DOI] [PubMed] [Google Scholar]