Abstract
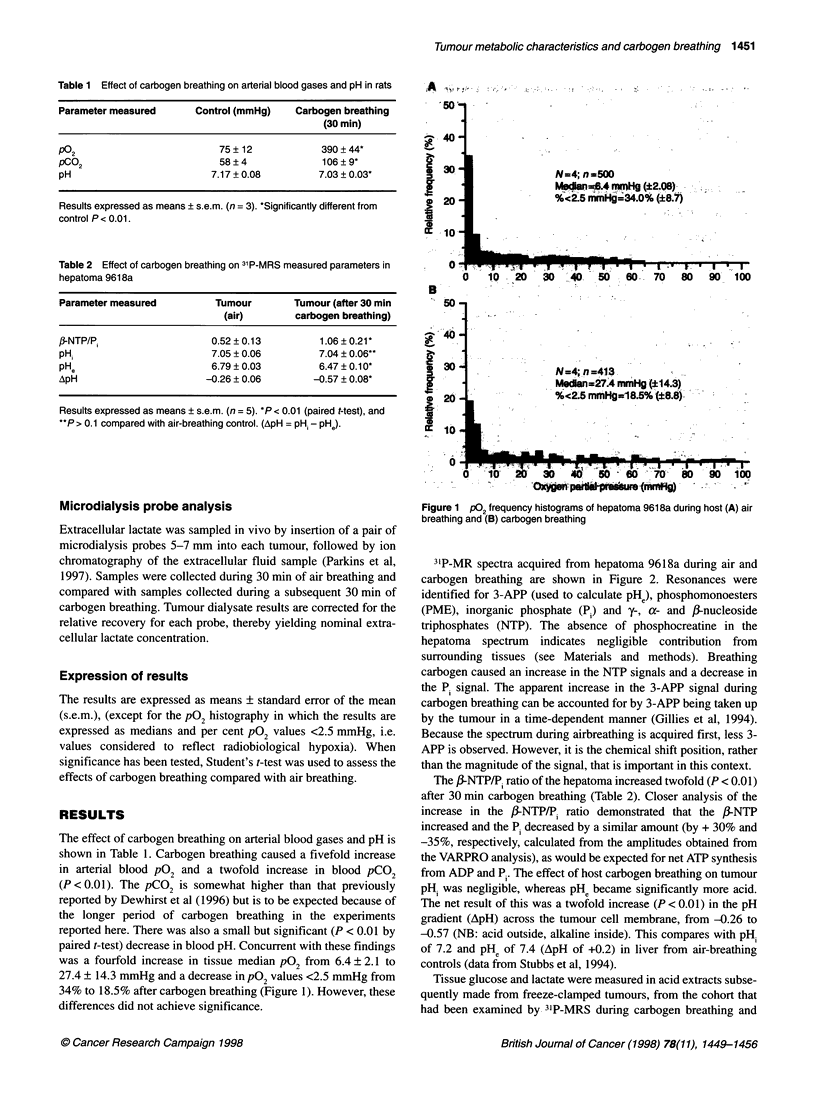
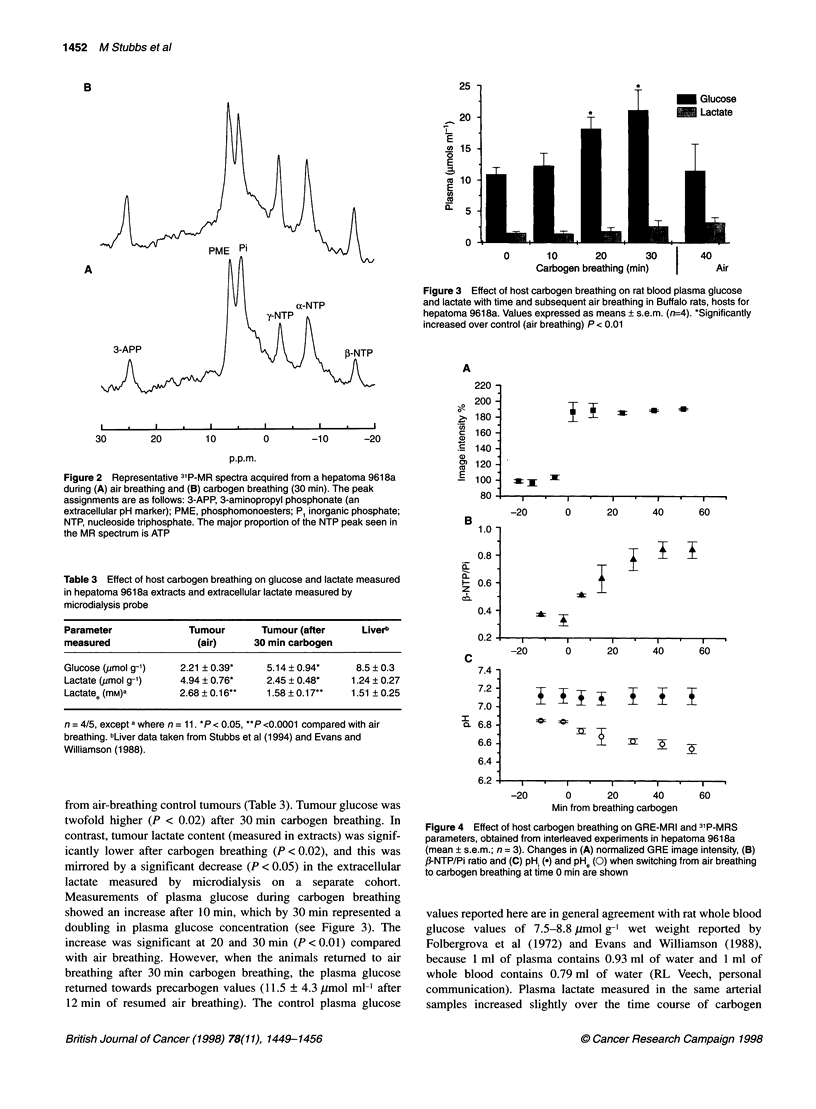
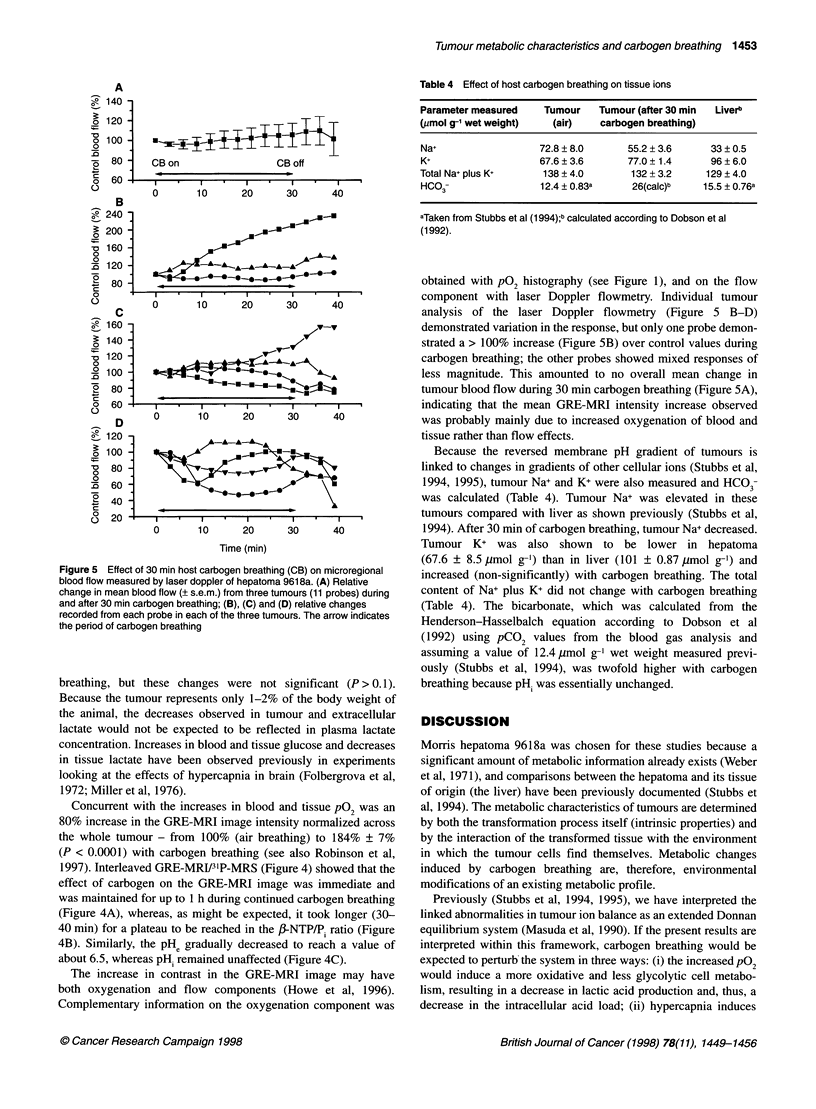
Characteristics of the tumour metabolic profile play a role in both the tumour-host interaction and in resistance to treatment. Because carbogen (95% oxygen/5% carbon dioxide) breathing can both increase sensitivity to radiation and improve chemotherapeutic efficacy, we have studied its effects on the metabolic characteristics of Morris hepatoma 9618a. Host carbogen breathing increased both arterial blood pCO2 and pO2, but decreased blood pH. A fourfold increase in tumour pO2 (measured polarographically) and a twofold increase in image intensity [measured by gradient recalled echo magnetic resonance (MR) imaging sensitive to changes in oxy/deoxyhaemoglobin] were observed. No changes were seen in blood flow measured by laser Doppler flowmetry. Tumour intracellular pH remained neutral, whereas extracellular pH decreased significantly (P < 0.01). Nucleoside triphosphate/inorganic phosphate (NTP/Pi), tissue and plasma glucose increased twofold and lactate decreased in both intra- and extracellular compartments, suggesting a change to a more oxidative metabolism. The improvement in energy status of the tumour was reflected in changes in tissue ions, including Na+, through ionic equilibria. The findings suggest that the metabolic profile of hepatoma 9618a is defined partly by intrinsic tumour properties caused by transformation and partly by tissue hypoxia, but that it can respond to environmental changes induced by carbogen with implications for improvements in therapeutic efficacy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brizel D. M., Lin S., Johnson J. L., Brooks J., Dewhirst M. W., Piantadosi C. A. The mechanisms by which hyperbaric oxygen and carbogen improve tumour oxygenation. Br J Cancer. 1995 Nov;72(5):1120–1124. doi: 10.1038/bjc.1995.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Hill S. A. Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumours. Br J Cancer. 1995 Jun;71(6):1210–1213. doi: 10.1038/bjc.1995.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst M. W., Ong E. T., Rosner G. L., Rehmus S. W., Shan S., Braun R. D., Brizel D. M., Secomb T. W. Arteriolar oxygenation in tumour and subcutaneous arterioles: effects of inspired air oxygen content. Br J Cancer Suppl. 1996 Jul;27:S241–S246. [PMC free article] [PubMed] [Google Scholar]
- Dobson G. P., Veech R. L., Passonneau J. V., Kobayashi K., Inubushi T., Wehrli S., Nioka S., Chance B. 31P NMR and enzymatic analysis of cytosolic phosphocreatine, ATP, Pi and intracellular pH in the isolated working perfused rat heart. NMR Biomed. 1992 Jan-Feb;5(1):20–28. doi: 10.1002/nbm.1940050105. [DOI] [PubMed] [Google Scholar]
- Evans R. D., Williamson D. H. Tissue-specific effects of rapid tumour growth on lipid metabolism in the rat during lactation and on litter removal. Biochem J. 1988 May 15;252(1):65–72. doi: 10.1042/bj2520065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S. J., Ward R., Bleehen N. M. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised p02 histography. Br J Cancer. 1992 Nov;66(5):919–924. doi: 10.1038/bjc.1992.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrová J., MacMillan V., Siesjö B. K. The effect of hypercapnic acidosis upon some glycolytic and Krebs cycle-associated intermediates in the rat brain. J Neurochem. 1972 Nov;19(11):2507–2517. doi: 10.1111/j.1471-4159.1972.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A., Gawlinski E. T. A reaction-diffusion model of cancer invasion. Cancer Res. 1996 Dec 15;56(24):5745–5753. [PubMed] [Google Scholar]
- Gatenby R. A. The potential role of transformation-induced metabolic changes in tumor-host interaction. Cancer Res. 1995 Sep 15;55(18):4151–4156. [PubMed] [Google Scholar]
- Gerweck L. E., Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996 Mar 15;56(6):1194–1198. [PubMed] [Google Scholar]
- Gillies R. J., Liu Z., Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994 Jul;267(1 Pt 1):C195–C203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- Grau C., Horsman M. R., Overgaard J. Improving the radiation response in a C3H mouse mammary carcinoma by normobaric oxygen or carbogen breathing. Int J Radiat Oncol Biol Phys. 1992;22(3):415–419. doi: 10.1016/0360-3016(92)90844-8. [DOI] [PubMed] [Google Scholar]
- Hill S. A., Collingridge D. R., Vojnovic B., Chaplin D. J. Tumour radiosensitization by high-oxygen-content gases: influence of the carbon dioxide content of the inspired gas on PO2, microcirculatory function and radiosensitivity. Int J Radiat Oncol Biol Phys. 1998 Mar 1;40(4):943–951. doi: 10.1016/s0360-3016(97)00892-4. [DOI] [PubMed] [Google Scholar]
- Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996 Oct 1;56(19):4509–4515. [PubMed] [Google Scholar]
- Honess D. J., Bleehen N. M. Perfusion changes in the RIF-1 tumour and normal tissues after carbogen and nicotinamide, individually and combined. Br J Cancer. 1995 Jun;71(6):1175–1180. doi: 10.1038/bjc.1995.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe F. A., Robinson S. P., Griffiths J. R. Modification of tumour perfusion and oxygenation monitored by gradient recalled echo MRI and 31P MRS. NMR Biomed. 1996 Aug;9(5):208–216. doi: 10.1002/(SICI)1099-1492(199608)9:5<208::AID-NBM418>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Schlenger K. H., Runkel S., Kloes M., Stohrer M., Okunieff P., Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989 Jul 15;49(14):3759–3764. [PubMed] [Google Scholar]
- Masuda T., Dobson G. P., Veech R. L. The Gibbs-Donnan near-equilibrium system of heart. J Biol Chem. 1990 Nov 25;265(33):20321–20334. [PubMed] [Google Scholar]
- McCoy C. L., Parkins C. S., Chaplin D. J., Griffiths J. R., Rodrigues L. M., Stubbs M. The effect of blood flow modification on intra- and extracellular pH measured by 31P magnetic resonance spectroscopy in murine tumours. Br J Cancer. 1995 Oct;72(4):905–911. doi: 10.1038/bjc.1995.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSheehy P. M., Robinson S. P., Ojugo A. S., Aboagye E. O., Cannell M. B., Leach M. O., Judson I. R., Griffiths J. R. Carbogen breathing increases 5-fluorouracil uptake and cytotoxicity in hypoxic murine RIF-1 tumors: a magnetic resonance study in vivo. Cancer Res. 1998 Mar 15;58(6):1185–1194. [PubMed] [Google Scholar]
- Menke H., Vaupel P. Effect of injectable or inhalational anesthetics and of neuroleptic, neuroleptanalgesic, and sedative agents on tumor blood flow. Radiat Res. 1988 Apr;114(1):64–76. [PubMed] [Google Scholar]
- Miller A. L., Hawkins R. A., Veech R. L. Decreased rate of glucose utilization by rat brain in vivo after exposure to atmospheres containing high concentrations of CO2. J Neurochem. 1975 Nov;25(5):553–558. doi: 10.1111/j.1471-4159.1975.tb04367.x. [DOI] [PubMed] [Google Scholar]
- Nordsmark M., Grau C., Horsman M. R., Jörgensen H. S., Overgaard J. Relationship between tumour oxygenation, bioenergetic status and radiobiological hypoxia in an experimental model. Acta Oncol. 1995;34(3):329–334. doi: 10.3109/02841869509093984. [DOI] [PubMed] [Google Scholar]
- Nordsmark M., Maxwell R. J., Horsman M. R., Bentzen S. M., Overgaard J. The effect of hypoxia and hyperoxia on nucleoside triphosphate/inorganic phosphate, pO2 and radiation response in an experimental tumour model. Br J Cancer. 1997;76(11):1432–1439. doi: 10.1038/bjc.1997.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins C. S., Stratford M. R., Dennis M. F., Stubbs M., Chaplin D. J. The relationship between extracellular lactate and tumour pH in a murine tumour model of ischaemia-reperfusion. Br J Cancer. 1997;75(3):319–323. doi: 10.1038/bjc.1997.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. E., Hill S. A., Saunders M. I., Hoskin P. J., Chaplin D. J. Effect of carbogen breathing on tumour microregional blood flow in humans. Radiother Oncol. 1996 Dec;41(3):225–231. doi: 10.1016/s0167-8140(96)01833-6. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Howe F. A., Griffiths J. R. Noninvasive monitoring of carbogen-induced changes in tumor blood flow and oxygenation by functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1995 Nov 1;33(4):855–859. doi: 10.1016/0360-3016(95)00072-1. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Rodrigues L. M., Ojugo A. S., McSheehy P. M., Howe F. A., Griffiths J. R. The response to carbogen breathing in experimental tumour models monitored by gradient-recalled echo magnetic resonance imaging. Br J Cancer. 1997;75(7):1000–1006. doi: 10.1038/bjc.1997.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L. M., Maxwell R. J., McSheehy P. M., Pinkerton C. R., Robinson S. P., Stubbs M., Griffiths J. R. In vivo detection of ifosfamide by 31P-MRS in rat tumours: increased uptake and cytotoxicity induced by carbogen breathing in GH3 prolactinomas. Br J Cancer. 1997;75(1):62–68. doi: 10.1038/bjc.1997.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A. Radiosensitization with normobaric oxygen and carbogen. Radiother Oncol. 1991;20 (Suppl 1):65–70. doi: 10.1016/0167-8140(91)90190-r. [DOI] [PubMed] [Google Scholar]
- Sansom J. M., Wood P. J. 31P MRS of tumour metabolism in anaesthetized vs conscious mice. NMR Biomed. 1994 Jun;7(4):167–171. doi: 10.1002/nbm.1940070403. [DOI] [PubMed] [Google Scholar]
- Schwickert G., Walenta S., Sundfør K., Rofstad E. K., Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995 Nov 1;55(21):4757–4759. [PubMed] [Google Scholar]
- Song C. W., Lee I., Hasegawa T., Rhee J. G., Levitt S. H. Increase in pO2 and radiosensitivity of tumors by Fluosol-DA (20%) and carbogen. Cancer Res. 1987 Jan 15;47(2):442–446. [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L., Howe F. A., Wang J., Jeong K. S., Veech R. L., Griffiths J. R. Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res. 1994 Aug 1;54(15):4011–4016. [PubMed] [Google Scholar]
- Stubbs M., Veech R. L., Griffiths J. R. Tumor metabolism: the lessons of magnetic resonance spectroscopy. Adv Enzyme Regul. 1995;35:101–115. doi: 10.1016/0065-2571(94)00016-v. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Schaefer C., Okunieff P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. 1994 May;7(3):128–136. doi: 10.1002/nbm.1940070305. [DOI] [PubMed] [Google Scholar]
- Veech R. L. The metabolism of lactate. NMR Biomed. 1991 Apr;4(2):53–58. doi: 10.1002/nbm.1940040204. [DOI] [PubMed] [Google Scholar]
- Weber G., Stubbs M., Morris H. P. Metabolism of hepatomas of different growth rates in situ and during ischemia. Cancer Res. 1971 Dec;31(12):2177–2183. [PubMed] [Google Scholar]
- van der Veen J. W., de Beer R., Luyten P. R., van Ormondt D. Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 1988 Jan;6(1):92–98. doi: 10.1002/mrm.1910060111. [DOI] [PubMed] [Google Scholar]


