Abstract
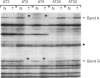
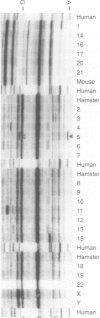
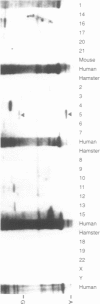
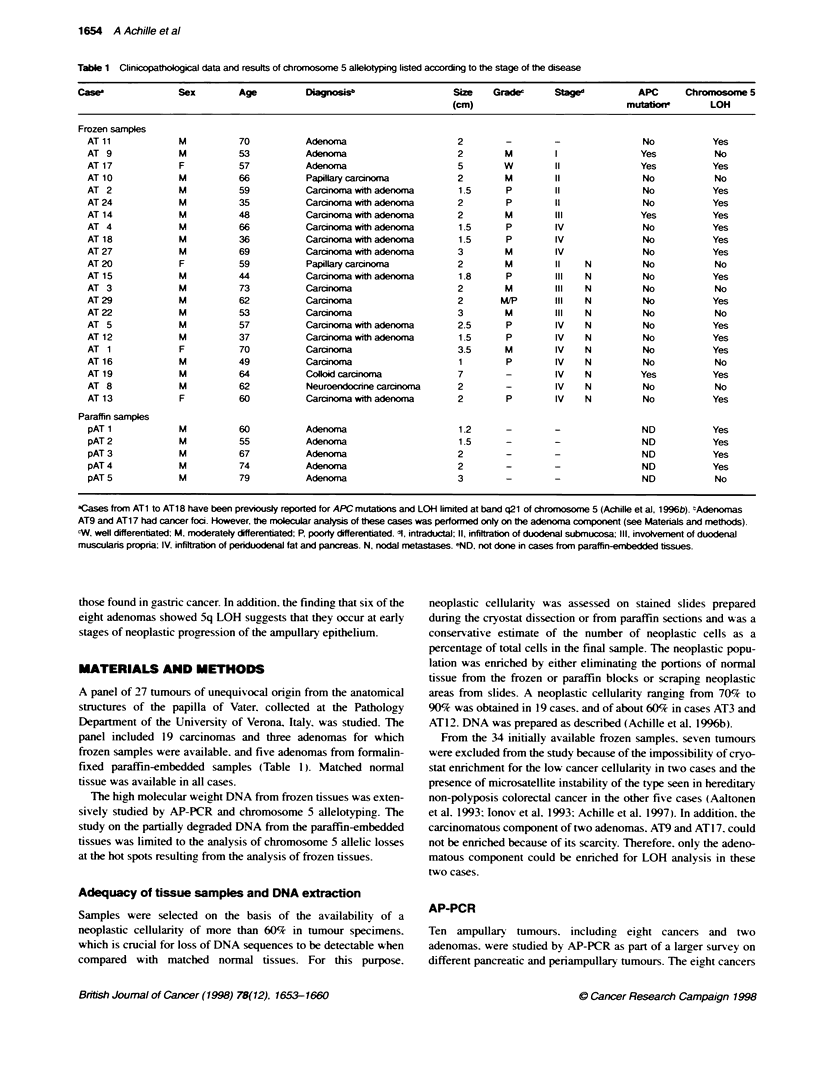
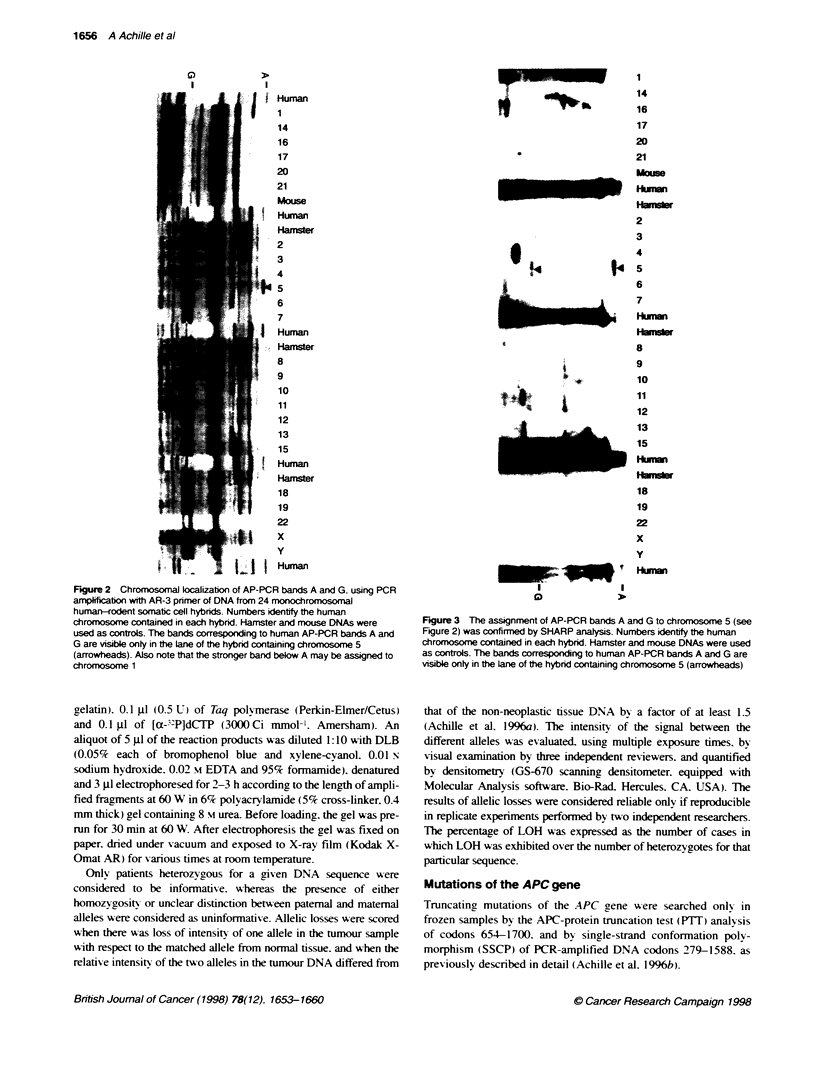
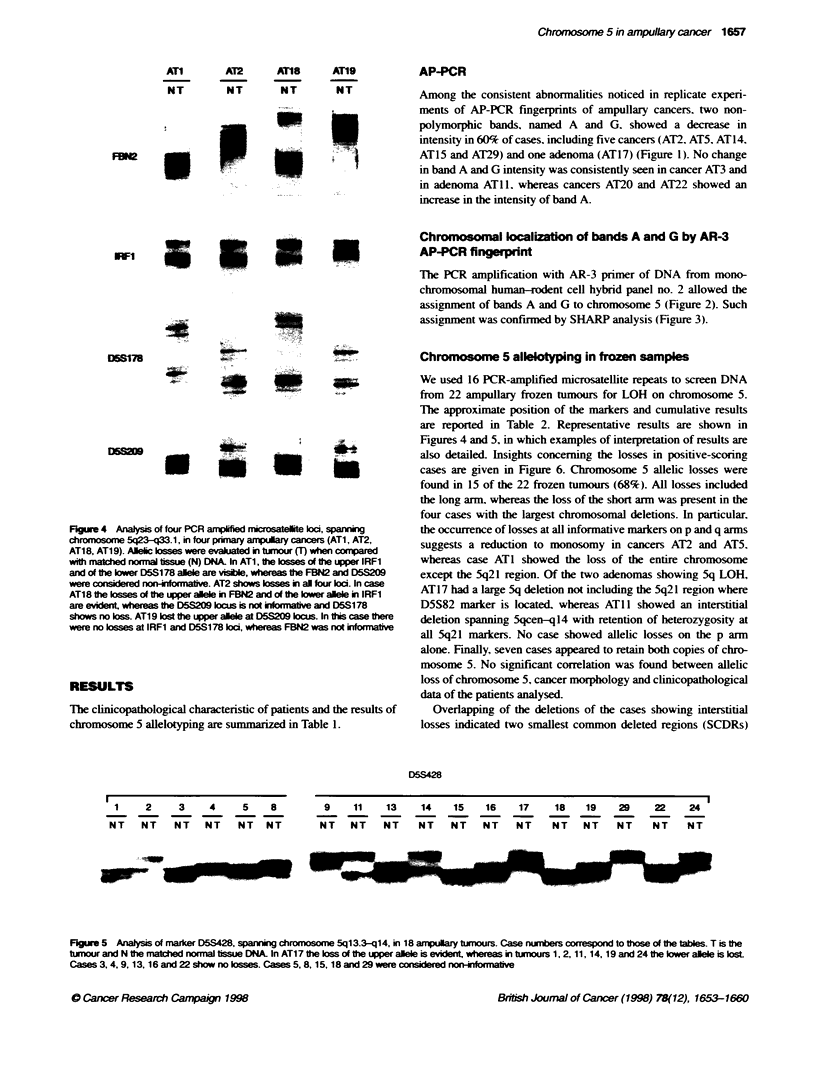
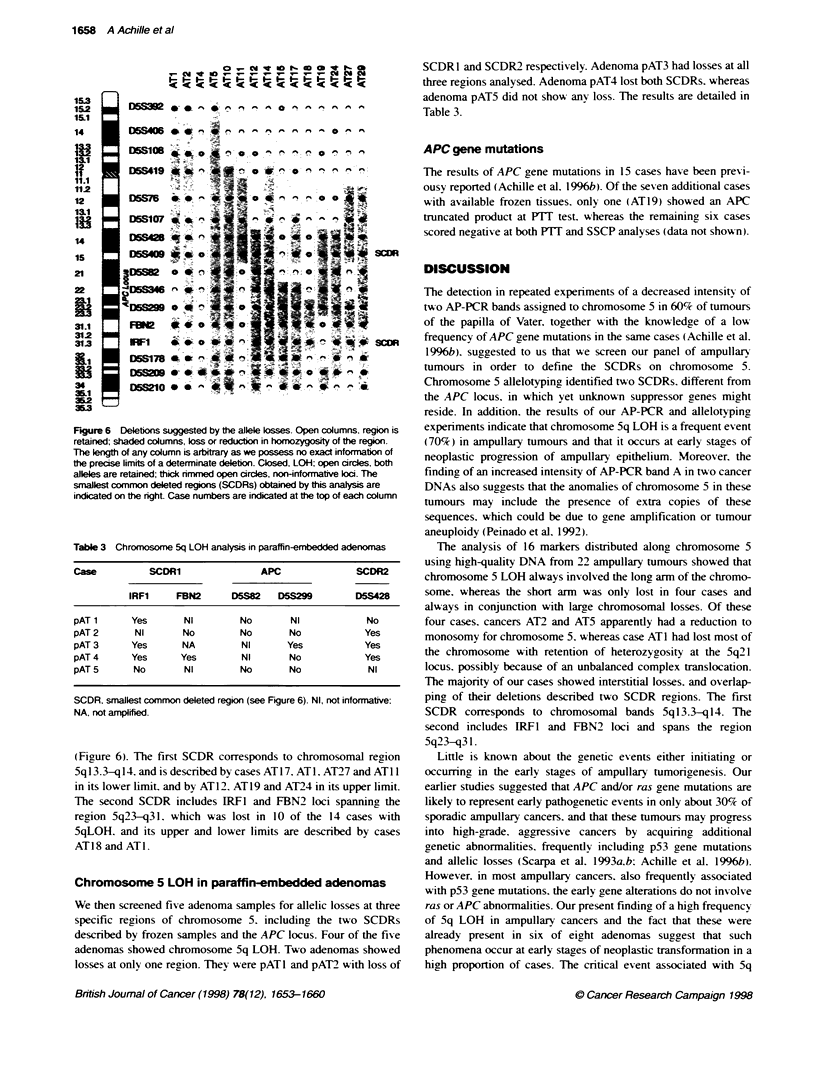
During our studies of DNA fingerprinting of tumours of the pancreas and papilla (ampulla) of Vater, using arbitrarily primed polymerase chain reaction (AP-PCR), we noticed two bands showing a decreased intensity in six of ten ampullary tumours with respect to matched normal tissues. Those bands were both assigned to chromosome 5. Such a finding was somewhat in contrast with the reportedly low frequency of APC gene mutations in ampullary cancers, located at chromosome 5q21, and suggested that loci different from that of APC might be the target of chromosome 5 allelic losses (LOH) in these tumours. Therefore, we analysed chromosome 5 LOH in a panel of 27 ampullary tumours, including eight adenomas, four early- and 15 advanced-stage cancers, using 16 PCR-amplified CA microsatellite polymorphic markers spanning the entire chromosome. Nineteen cases (70%) showed LOH, and the interstitial deletions found in these tumours described two smallest common deleted regions, in which putative suppressor genes might reside. They were at 5q13.3-q14 and at 5q23-q31 respectively, which correspond to those found in gastric tumours. In addition, the presence of 5q LOH in six of eight adenomas and in three of four early-stage cancers suggests that such phenomena occur at early stages of neoplastic progression of the ampullary epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Achille A., Biasi M. O., Zamboni G., Bogina G., Iacono C., Talamini G., Capella G., Scarpa A. Cancers of the papilla of vater: mutator phenotype is associated with good prognosis. Clin Cancer Res. 1997 Oct;3(10):1841–1847. [PubMed] [Google Scholar]
- Achille A., Biasi M. O., Zamboni G., Bogina G., Magalini A. R., Pederzoli P., Perucho M., Scarpa A. Chromosome 7q allelic losses in pancreatic carcinoma. Cancer Res. 1996 Aug 15;56(16):3808–3813. [PubMed] [Google Scholar]
- Achille A., Scupoli M. T., Magalini A. R., Zamboni G., Romanelli M. G., Orlandini S., Biasi M. O., Lemoine N. R., Accolla R. S., Scarpa A. APC gene mutations and allelic losses in sporadic ampullary tumours: evidence of genetic difference from tumours associated with familial adenomatous polyposis. Int J Cancer. 1996 Nov 4;68(3):305–312. doi: 10.1002/(SICI)1097-0215(19961104)68:3<305::AID-IJC7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bardi G., Aman P., Johansson B., Pandis N., Mandahl N., Bak-Jensen E., Björkman A., Sjögren H. O., Andrén-Sandberg A., Mitelman F. Cytogenetic characterization of a periampullary adenocarcinoma of the pancreas, its liver metastasis, and a cell line established from the metastasis and a cell line established from the metastasis in a patient with Gardner's syndrome. Cancer Genet Cytogenet. 1994 Aug;76(1):29–32. doi: 10.1016/0165-4608(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Bardi G., Johansson B., Pandis N., Mandahl N., Bak-Jensen E., Andrén-Sandberg A., Mitelman F., Heim S. Karyotypic abnormalities in tumours of the pancreas. Br J Cancer. 1993 May;67(5):1106–1112. doi: 10.1038/bjc.1993.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwinga H. L., Toji L. H., Kim C. H., Greene A. E., Mulivor R. A. NIGMS human/rodent somatic cell hybrid mapping panels 1 and 2. Genomics. 1993 May;16(2):311–314. doi: 10.1006/geno.1993.1190. [DOI] [PubMed] [Google Scholar]
- Hahn S. A., Seymour A. B., Hoque A. T., Schutte M., da Costa L. T., Redston M. S., Caldas C., Weinstein C. L., Fischer A., Yeo C. J. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995 Oct 15;55(20):4670–4675. [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Ando H., Yanagisawa A., Tsuchiya E., Kato Y., Nakamura Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992 Dec 1;52(23):6696–6698. [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Kato Y., Yanagisawa A., Nakamura Y. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res. 1992 Jun 1;52(11):3231–3233. [PubMed] [Google Scholar]
- Horrigan S. K., Westbrook C. A., Kim A. H., Banerjee M., Stock W., Larson R. A. Polymerase chain reaction-based diagnosis of del (5q) in acute myeloid leukemia and myelodysplastic syndrome identifies a minimal deletion interval. Blood. 1996 Oct 1;88(7):2665–2670. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jen J., Powell S. M., Papadopoulos N., Smith K. J., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994 Nov 1;54(21):5523–5526. [PubMed] [Google Scholar]
- Johansson B., Bardi G., Heim S., Mandahl N., Mertens F., Bak-Jensen E., Andrén-Sandberg A., Mitelman F. Nonrandom chromosomal rearrangements in pancreatic carcinomas. Cancer. 1992 Apr 1;69(7):1674–1681. doi: 10.1002/1097-0142(19920401)69:7<1674::aid-cncr2820690706>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996 Oct 18;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Klempnauer J., Ridder G. J., Pichlmayr R. Prognostic factors after resection of ampullary carcinoma: multivariate survival analysis in comparison with ductal cancer of the pancreatic head. Br J Surg. 1995 Dec;82(12):1686–1691. doi: 10.1002/bjs.1800821233. [DOI] [PubMed] [Google Scholar]
- Louis D. N., von Deimling A., Seizinger B. R. A (CA)n dinucleotide repeat assay for evaluating loss of allelic heterozygosity in small and archival human brain tumor specimens. Am J Pathol. 1992 Oct;141(4):777–782. [PMC free article] [PubMed] [Google Scholar]
- McKie A. B., Filipe M. I., Lemoine N. R. Abnormalities affecting the APC and MCC tumour suppressor gene loci on chromosome 5q occur frequently in gastric cancer but not in pancreatic cancer. Int J Cancer. 1993 Oct 21;55(4):598–603. doi: 10.1002/ijc.2910550414. [DOI] [PubMed] [Google Scholar]
- Murty V. V., Reuter V. E., Bosl G. J., Chaganti R. S. Deletion mapping identifies loss of heterozygosity at 5p15.1-15.2, 5q11 and 5q34-35 in human male germ cell tumors. Oncogene. 1996 Jun 20;12(12):2719–2723. [PubMed] [Google Scholar]
- Peinado M. A., Malkhosyan S., Velazquez A., Perucho M. Isolation and characterization of allelic losses and gains in colorectal tumors by arbitrarily primed polymerase chain reaction. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10065–10069. doi: 10.1073/pnas.89.21.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. M., Papadopoulos N., Kinzler K. W., Smolinski K. N., Meltzer S. J. APC gene mutations in the mutation cluster region are rare in esophageal cancers. Gastroenterology. 1994 Dec;107(6):1759–1763. doi: 10.1016/0016-5085(94)90818-4. [DOI] [PubMed] [Google Scholar]
- Rose D. M., Hochwald S. N., Klimstra D. S., Brennan M. F. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996 Aug;183(2):89–96. [PubMed] [Google Scholar]
- Scarpa A., Capelli P., Mukai K., Zamboni G., Oda T., Iacono C., Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993 May;142(5):1534–1543. [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Capelli P., Zamboni G., Oda T., Mukai K., Bonetti F., Martignoni G., Iacono C., Serio G., Hirohashi S. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993 Apr;142(4):1163–1172. [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Zamboni G., Achille A., Capelli P., Bogina G., Iacono C., Serio G., Accolla R. S. ras-family gene mutations in neoplasia of the ampulla of Vater. Int J Cancer. 1994 Oct 1;59(1):39–42. doi: 10.1002/ijc.2910590109. [DOI] [PubMed] [Google Scholar]
- Seymour A. B., Hruban R. H., Redston M., Caldas C., Powell S. M., Kinzler K. W., Yeo C. J., Kern S. E. Allelotype of pancreatic adenocarcinoma. Cancer Res. 1994 May 15;54(10):2761–2764. [PubMed] [Google Scholar]
- Tamura G., Maesawa C., Suzuki Y., Tamada H., Satoh M., Ogasawara S., Kashiwaba M., Satodate R. Mutations of the APC gene occur during early stages of gastric adenoma development. Cancer Res. 1994 Mar 1;54(5):1149–1151. [PubMed] [Google Scholar]
- Tamura G., Ogasawara S., Nishizuka S., Sakata K., Maesawa C., Suzuki Y., Terashima M., Saito K., Satodate R. Two distinct regions of deletion on the long arm of chromosome 5 in differentiated adenocarcinomas of the stomach. Cancer Res. 1996 Feb 1;56(3):612–615. [PubMed] [Google Scholar]
- Tavassoli M., Steingrimsdottir H., Pierce E., Jiang X., Alagoz M., Farzaneh F., Campbell I. G. Loss of heterozygosity on chromosome 5q in ovarian cancer is frequently accompanied by TP53 mutation and identifies a tumour suppressor gene locus at 5q13.1-21. Br J Cancer. 1996 Jul;74(1):115–119. doi: 10.1038/bjc.1996.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I., Böhm M., Arden K. C., Ammermüller T., Bogatz S., Viars C. S., Rajewsky M. F. Allelic deletion mapping on chromosome 5 in human carcinomas. Oncogene. 1996 Jan 4;12(1):97–102. [PubMed] [Google Scholar]
- Willman C. L., Sever C. E., Pallavicini M. G., Harada H., Tanaka N., Slovak M. L., Yamamoto H., Harada K., Meeker T. C., List A. F. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science. 1993 Feb 12;259(5097):968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]
- Yashima K., Nakamori S., Murakami Y., Yamaguchi A., Hayashi K., Ishikawa O., Konishi Y., Sekiya T. Mutations of the adenomatous polyposis coli gene in the mutation cluster region: comparison of human pancreatic and colorectal cancers. Int J Cancer. 1994 Oct 1;59(1):43–47. doi: 10.1002/ijc.2910590110. [DOI] [PubMed] [Google Scholar]
- Yasuda J., Navarro J. M., Malkhosyan S., Velazquez A., Arribas R., Sekiya T., Perucho M. Chromosomal assignment of human DNA fingerprint sequences by simultaneous hybridization to arbitrarily primed PCR products from human/rodent monochromosome cell hybrids. Genomics. 1996 May 15;34(1):1–8. doi: 10.1006/geno.1996.0235. [DOI] [PubMed] [Google Scholar]