Abstract
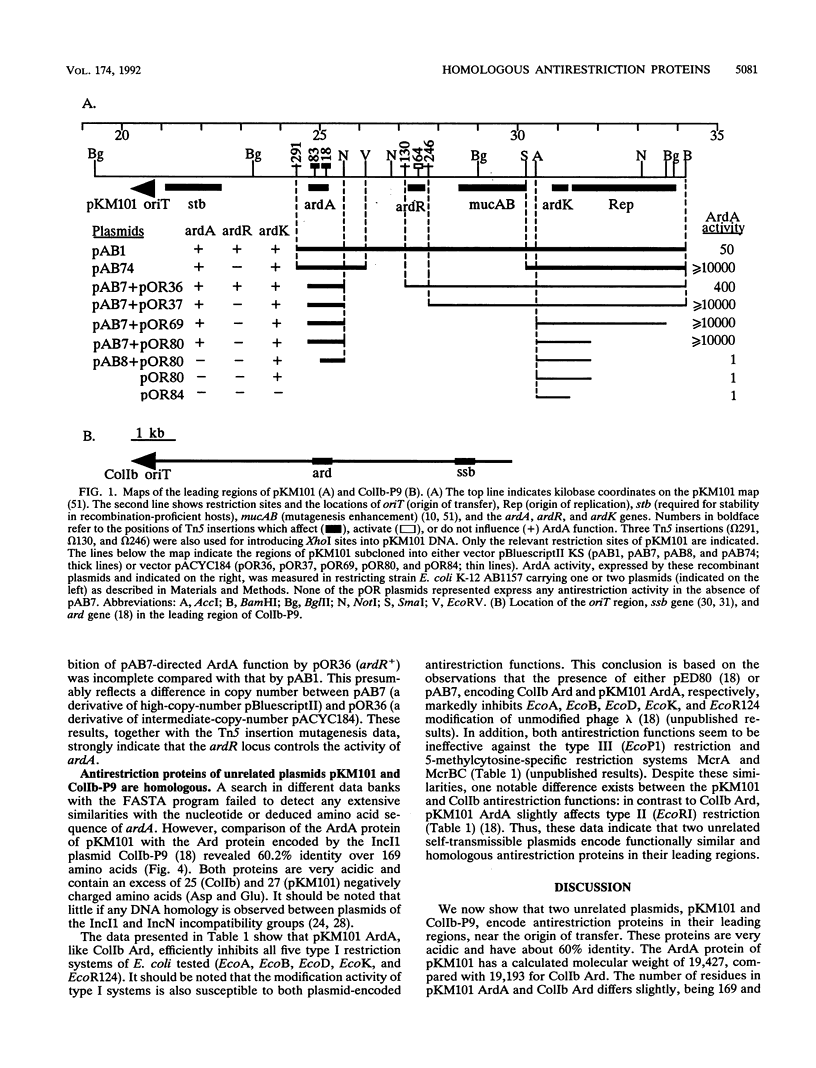
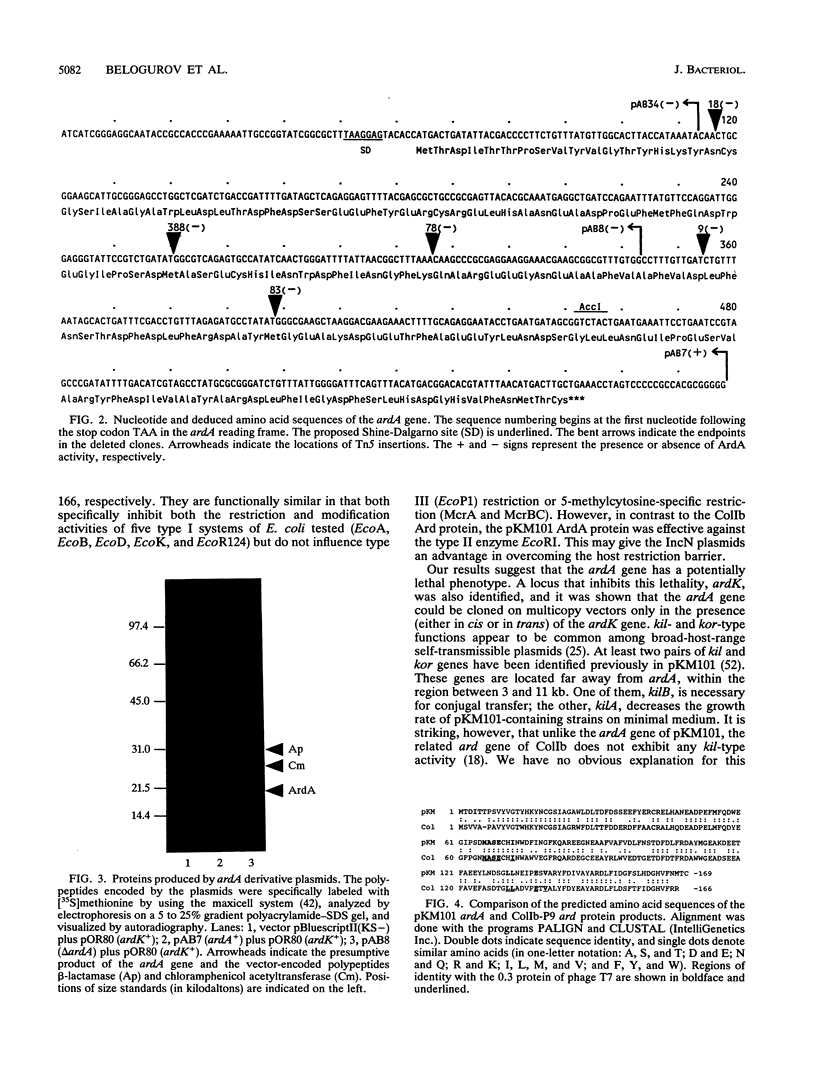
The IncN plasmid pKM101 (a derivative of R46), like the IncI1 plasmid ColIb-P9, carries a gene (ardA, for alleviation of restriction of DNA) encoding an antirestriction function. ardA was located about 4 kb from the origin of transfer, in the region transferred early during bacterial conjugation. The nucleotide sequence of ardA was determined, and an appropriate polypeptide with the predicted molecular weight of about 19,500 was identified in maxicells of Escherichia coli. Comparison of the deduced amino acid sequences of the antirestriction proteins of the unrelated plasmids pKM101 and ColIb (ArdA and Ard, respectively) revealed that these proteins have about 60% identity. Like ColIb Ard, pKM101 ArdA specifically inhibits both the restriction and modification activities of five type I systems of E. coli tested and does not influence type III (EcoP1) restriction or the 5-methylcytosine-specific restriction systems McrA and McrB. However, in contrast to ColIb Ard, pKM101 ArdA is effective against the type II enzyme EcoRI. The Ard proteins are believed to overcome the host restriction barrier during bacterial conjugation. We have also identified two other genes of pKM101, ardR and ardK, which seem to control ardA activity and ardA-mediated lethality, respectively. Our findings suggest that ardR may serve as a genetic switch that determines whether the ardA-encoded antirestriction function is induced during mating.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Bailone A., Bagdasarian M. M., Manning P. A., Lurz R., Timmis K. N., Devoret R. An inhibitor of SOS induction, specified by a plasmid locus in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5723–5726. doi: 10.1073/pnas.83.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay P. K., Studier F. W., Hamilton D. L., Yuan R. Inhibition of the type I restriction-modification enzymes EcoB and EcoK by the gene 0.3 protein of bacteriophage T7. J Mol Biol. 1985 Apr 20;182(4):567–578. doi: 10.1016/0022-2836(85)90242-6. [DOI] [PubMed] [Google Scholar]
- Belogurov A. A., Yussifov T. N., Kotova V. U., Zavilgelsky G. B. The novel gene(s) ARD of plasmid pKM101: alleviation of EcoK restriction. Mol Gen Genet. 1985;198(3):509–513. doi: 10.1007/BF00332948. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Willetts N. S. A physical and genetic map of the IncN plasmid R46. Plasmid. 1981 Mar;5(2):188–201. doi: 10.1016/0147-619x(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Colson C., Van Pel A. DNA restriction and modification systems in Salmonella. SQ, a new system derived by recombination between the SB system of Salmonella typhimurium and the SP system of Salmonella potsdam. J Gen Microbiol. 1976 Jul;95(1):166–172. doi: 10.1099/00221287-95-1-166. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland G. M., Brown A. M., Willetts N. S. The origin of transfer (oriT) of the conjugative plasmid R46: characterization by deletion analysis and DNA sequencing. Mol Gen Genet. 1987 Jun;208(1-2):219–225. doi: 10.1007/BF00330445. [DOI] [PubMed] [Google Scholar]
- Datta N. Plasmids as organisms. Basic Life Sci. 1985;30:3–16. doi: 10.1007/978-1-4613-2447-8_2. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd UV-induced alleviation of K-specific restriction of bacteriophage lambda. J Virol. 1977 Mar;21(3):1249–1251. doi: 10.1128/jvi.21.3.1249-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delver E. P., Kotova V. U., Zavilgelsky G. B., Belogurov A. A. Nucleotide sequence of the gene (ard) encoding the antirestriction protein of plasmid colIb-P9. J Bacteriol. 1991 Sep;173(18):5887–5892. doi: 10.1128/jb.173.18.5887-5892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmalingam K., Revel H. R., Goldberg E. B. Physical mapping and cloning of bacteriophage T4 anti-restriction endonuclease gene. J Bacteriol. 1982 Feb;149(2):694–699. doi: 10.1128/jb.149.2.694-699.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Elzinga M., Mark K. K., Studier F. W. Amino acid sequence of the gene 0.3 protein of bacteriophage T7 and nucleotide sequence of its mRNA. J Biol Chem. 1981 Mar 10;256(5):2579–2585. [PubMed] [Google Scholar]
- Efimova E. P., Delver E. P., Belogurov A. A. 2-Aminopurine and 5-bromouracil induce alleviation of type I restriction in Escherichia coli: mismatches function as inducing signals? Mol Gen Genet. 1988 Oct;214(2):317–320. doi: 10.1007/BF00337728. [DOI] [PubMed] [Google Scholar]
- Efimova E. P., Delver E. P., Belogurov A. A. Alleviation of type I restriction in adenine methylase (dam) mutants of Escherichia coli. Mol Gen Genet. 1988 Oct;214(2):313–316. doi: 10.1007/BF00337727. [DOI] [PubMed] [Google Scholar]
- Endlich B., Linn S. The DNA restriction endonuclease of Escherichia coli B. II. Further studies of the structure of DNA intermediates and products. J Biol Chem. 1985 May 10;260(9):5729–5738. [PubMed] [Google Scholar]
- Falkow S., Guerry P., Hedges R. W., Datta N. Polynucleotide sequence relationships among plasmids of the I compatibility complex. J Gen Microbiol. 1974 Nov;85(1):65–76. doi: 10.1099/00221287-85-1-65. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Pohlman R. F., Bechhofer D. H., Prince A. S., Kelton C. A. Broad host range plasmid RK2 encodes multiple kil genes potentially lethal to Escherichia coli host cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1935–1939. doi: 10.1073/pnas.79.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Bullas L. R., Delius H., Murray N. E. Genetic recombination can generate altered restriction specificity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6095–6099. doi: 10.1073/pnas.81.19.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann A. A., Campbell A. J., Collins J. F., Coulson A. F., Murray N. E. Reassortment of DNA recognition domains and the evolution of new specificities. Mol Microbiol. 1987 Jul;1(1):13–22. doi: 10.1111/j.1365-2958.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Humphreys G. O., Anderson E. S. Molecular studies of R factor compatibility groups. J Bacteriol. 1973 Jul;115(1):387–398. doi: 10.1128/jb.115.1.387-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Howland C. J., Rees C. E., Barth P. T., Wilkins B. M. The ssb gene of plasmid ColIb-P9. J Bacteriol. 1989 May;171(5):2466–2473. doi: 10.1128/jb.171.5.2466-2473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland C. J., Wilkins B. M. Direction of conjugative transfer of IncI1 plasmid ColIb-P9. J Bacteriol. 1988 Oct;170(10):4958–4959. doi: 10.1128/jb.170.10.4958-4959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Bickle T. A. Bacteriophage survival: multiple mechanisms for avoiding the deoxyribonucleic acid restriction systems of their hosts. Microbiol Rev. 1983 Sep;47(3):345–360. doi: 10.1128/mr.47.3.345-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Walker G. C. Restriction endonuclease cleavage map of pKM101: relationship to parental plasmid R46. Mol Gen Genet. 1981;182(2):268–272. doi: 10.1007/BF00269669. [DOI] [PubMed] [Google Scholar]
- Mark K. K., Studier F. W. Purification of the gene 0.3 protein of bacteriophage T7, an inhibitor of the DNA restriction system of Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2573–2578. [PubMed] [Google Scholar]
- Price C., Lingner J., Bickle T. A., Firman K., Glover S. W. Basis for changes in DNA recognition by the EcoR124 and EcoR124/3 type I DNA restriction and modification enzymes. J Mol Biol. 1989 Jan 5;205(1):115–125. doi: 10.1016/0022-2836(89)90369-0. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulland-Dussoix D., Boyer H. W. The Escherichia coli B restriction endonuclease. Biochim Biophys Acta. 1969 Nov 19;195(1):219–229. doi: 10.1016/0005-2787(69)90618-2. [DOI] [PubMed] [Google Scholar]
- Sain B., Murray N. E. The hsd (host specificity) genes of E. coli K 12. Mol Gen Genet. 1980;180(1):35–46. doi: 10.1007/BF00267350. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P., Bickle T. A. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature. 1979 Mar 1;278(5699):30–34. doi: 10.1038/278030a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Genetic control of damage-inducible restriction alleviation in Escherichia coli K12: an SOS function not repressed by lexA. Mol Gen Genet. 1984;197(2):297–303. doi: 10.1007/BF00330977. [DOI] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. UV-induced allevation of lambda restriction in Escherichia coli K-12: kinetics of induction and specificity of this SOS function. Mol Gen Genet. 1982;186(1):111–117. doi: 10.1007/BF00422921. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G. Organization of restriction-modification systems. Nucleic Acids Res. 1991 May 25;19(10):2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985 Jan;161(1):402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Identification of pKM101-encoded loci specifying potentially lethal gene products. J Bacteriol. 1985 Jan;161(1):417–424. doi: 10.1128/jb.161.1.417-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Friedman S., Van Montagu M., Schell J. The ral gene of phage lambda. I. Identification of a non-essential gene that modulates restriction and modification in E. coli. Mol Gen Genet. 1980;179(1):63–73. doi: 10.1007/BF00268447. [DOI] [PubMed] [Google Scholar]