Abstract
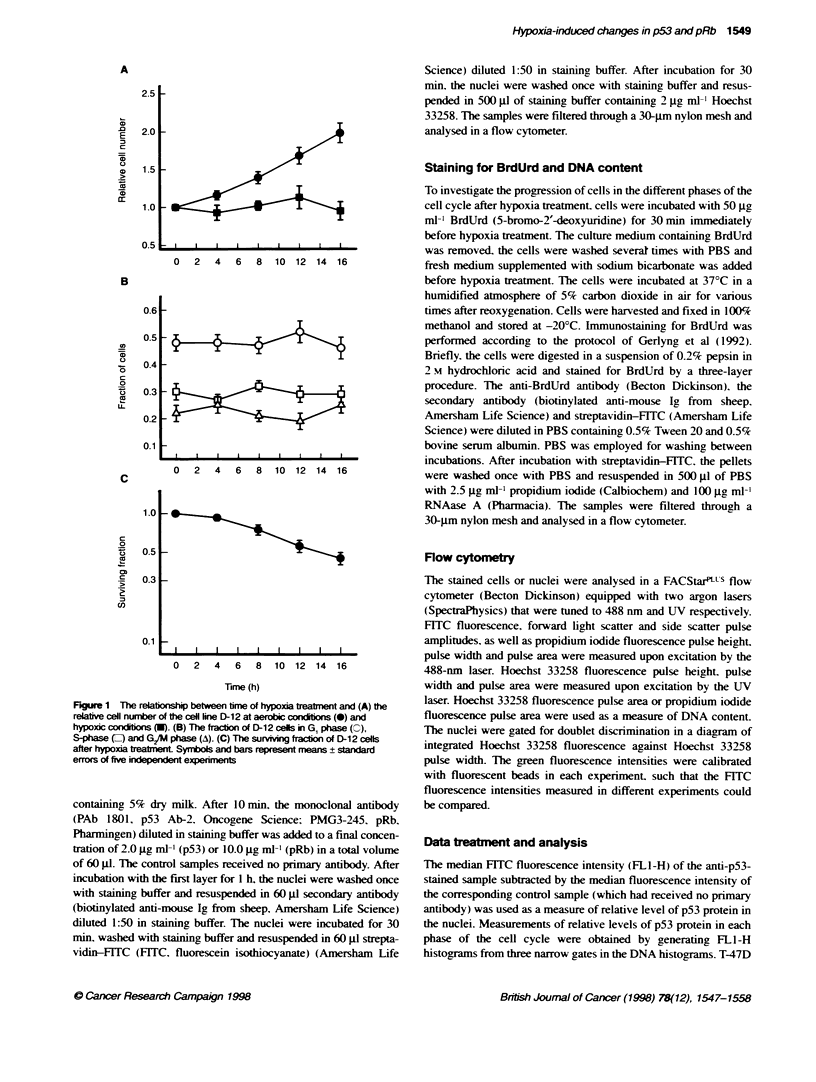
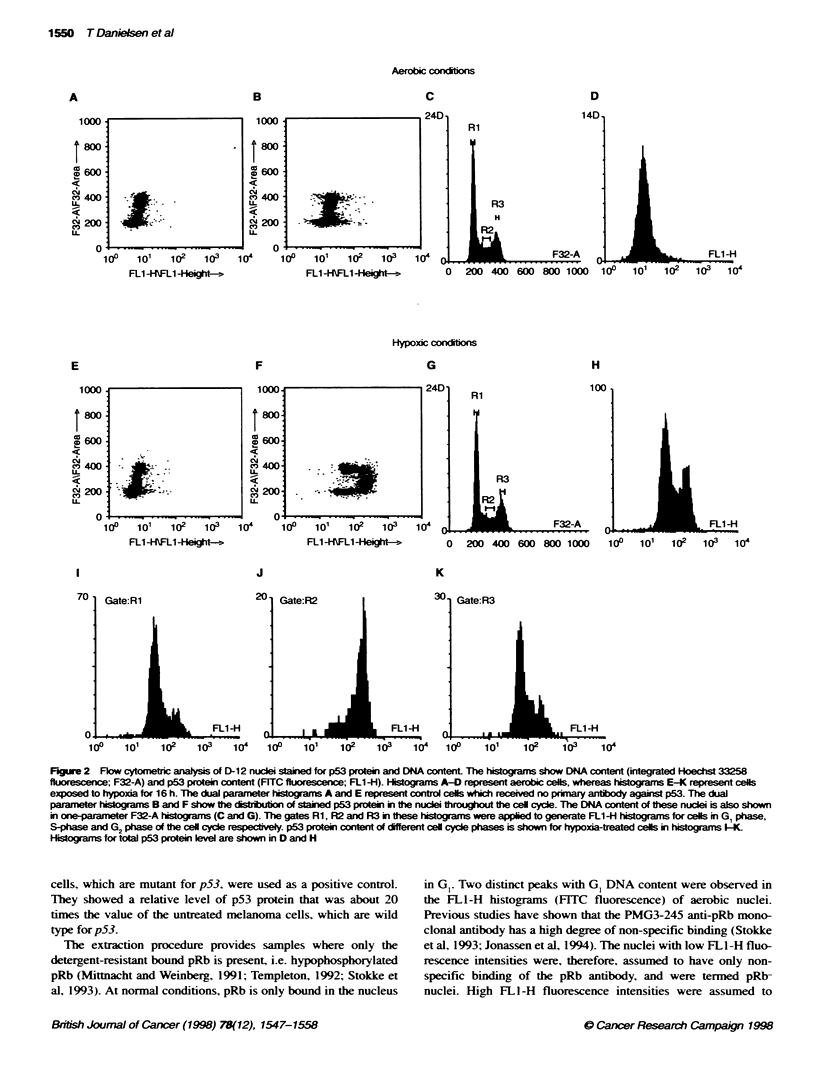
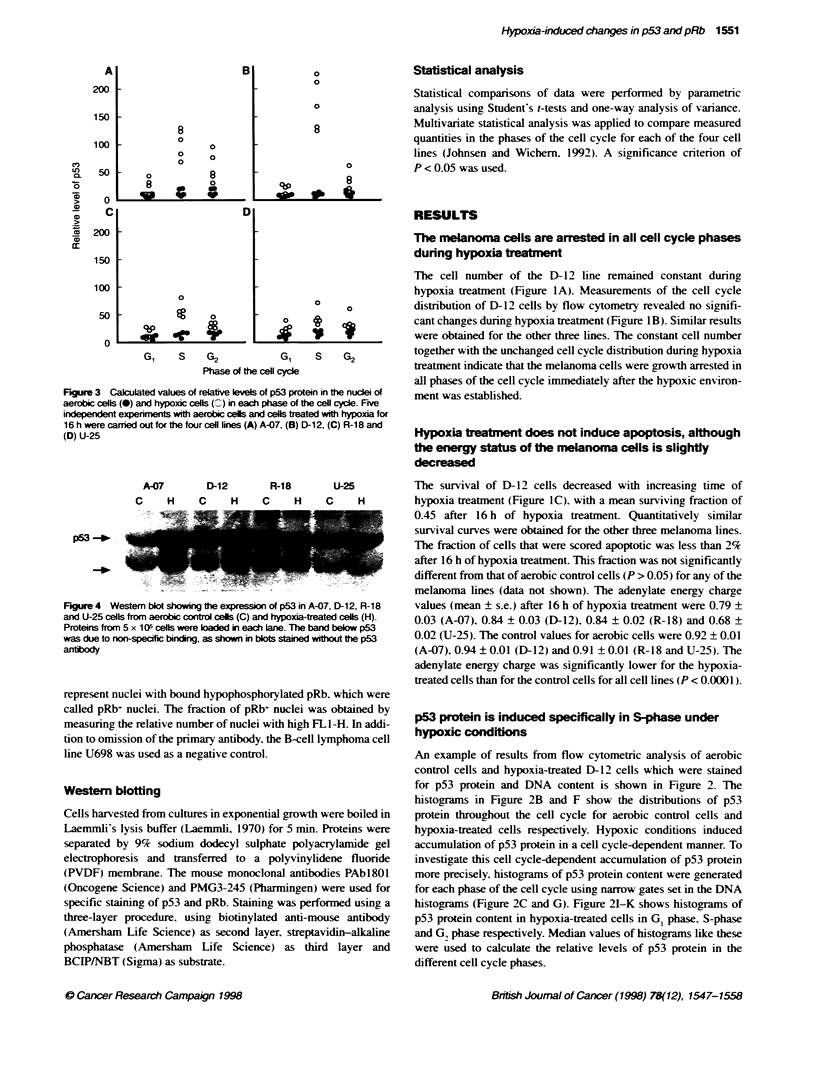
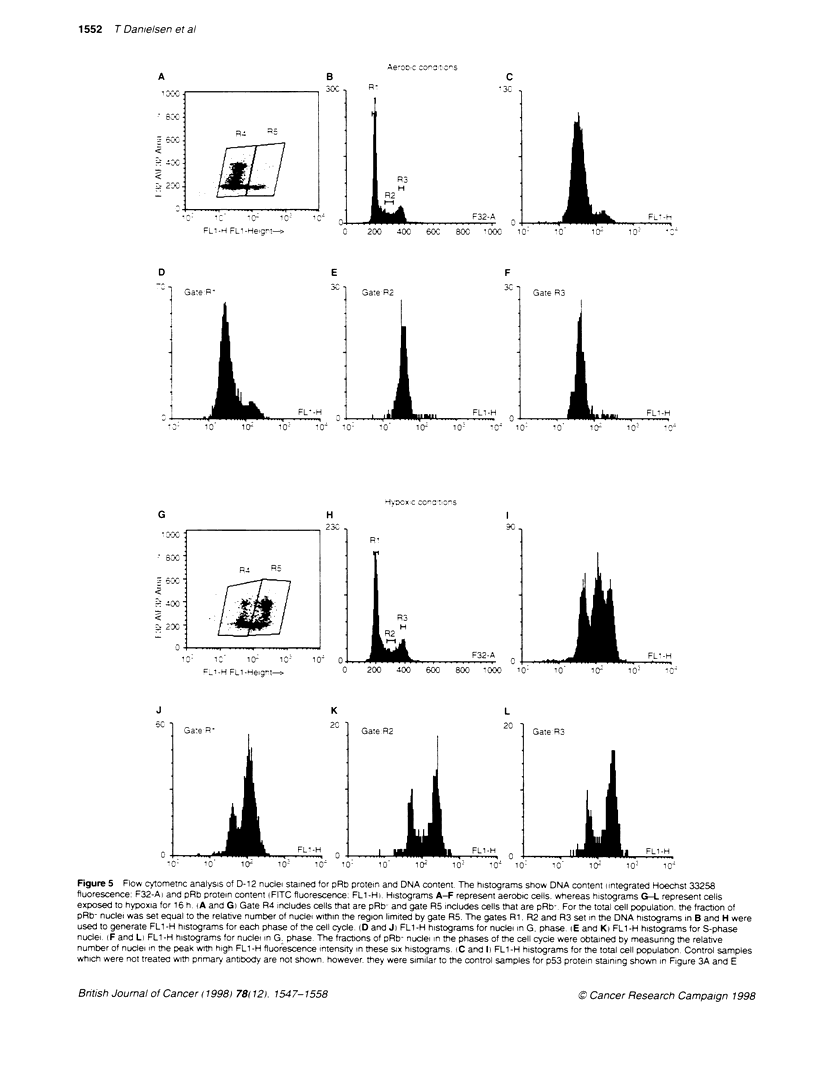
Hypoxia has been shown to induce accumulation of p53 and of hypophosphorylated retinoblastoma protein (pRb) in tumour cells. In this study, the cell cycle dependence of p53 accumulation and pRb hypophosphorylation in four human melanoma cell lines that are wild type for p53 was investigated using two-parameter flow cytometry measurements of p53 or pRb protein content and DNA content. The hypoxia-induced increase in p53 protein was higher in S-phase than in G1 and G2 phases in all cell lines. The accumulation of p53 in S-phase during hypoxia was not related to hypoxia-induced apoptosis or substantial cell cycle specific cell inactivation during the first 24 h of reoxygenation. pRb was hypophosphorylated in all cell cycle phases by hypoxia treatment. The results did not support a direct link between p53 and pRb during hypoxia because p53 was induced in a cell cycle-specific manner, whereas no cell cycle-dependent differences in pRb hypophosphorylation were detected. Only a fraction of the cell populations (0.60+/-0.10) showed hypophosphorylated pRb. Thus, pRb is probably not the only mediator of the hypoxia-induced cell cycle block seen in all cells and all cell cycle phases. Moreover, the cell cycle-dependent induction of p53 by hypoxia suggests that the primary function of p53 accumulation during hypoxia is other than to arrest the cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amellem O., Pettersen E. O. Cell inactivation and cell cycle inhibition as induced by extreme hypoxia: the possible role of cell cycle arrest as a protection against hypoxia-induced lethal damage. Cell Prolif. 1991 Mar;24(2):127–141. doi: 10.1111/j.1365-2184.1991.tb01144.x. [DOI] [PubMed] [Google Scholar]
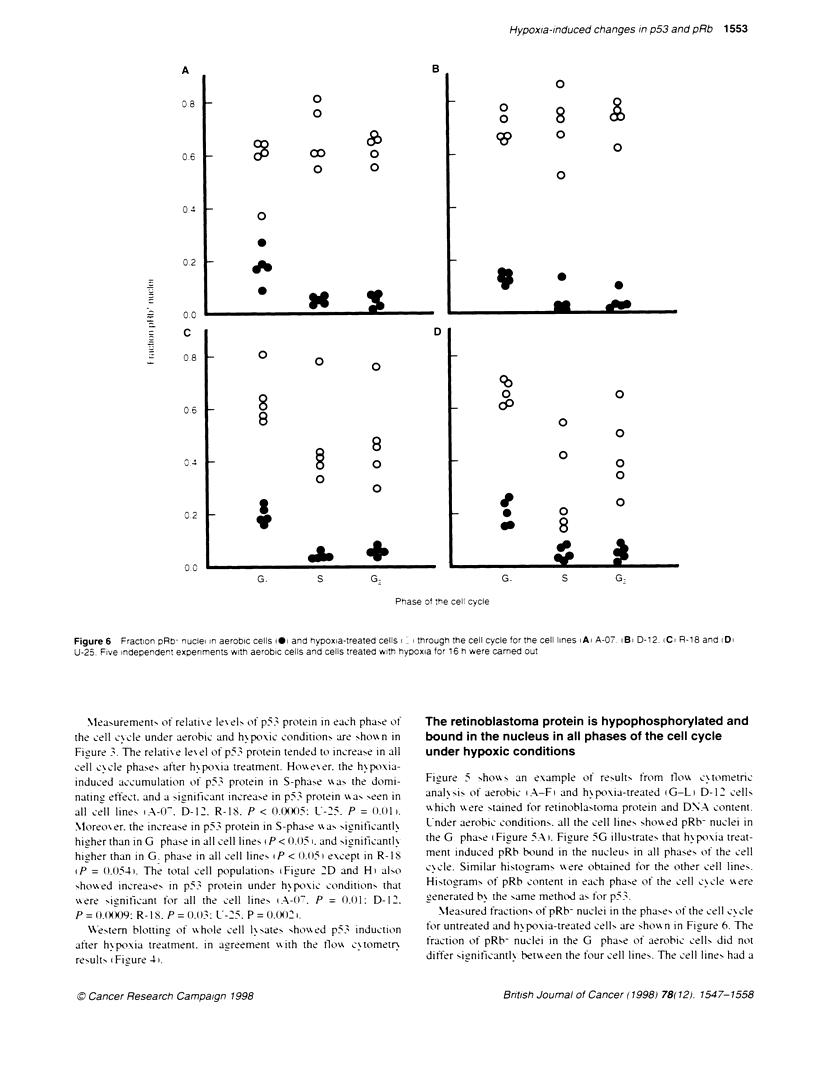
- Amellem O., Stokke T., Sandvik J. A., Pettersen E. O. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp Cell Res. 1996 Aug 25;227(1):106–115. doi: 10.1006/excr.1996.0255. [DOI] [PubMed] [Google Scholar]
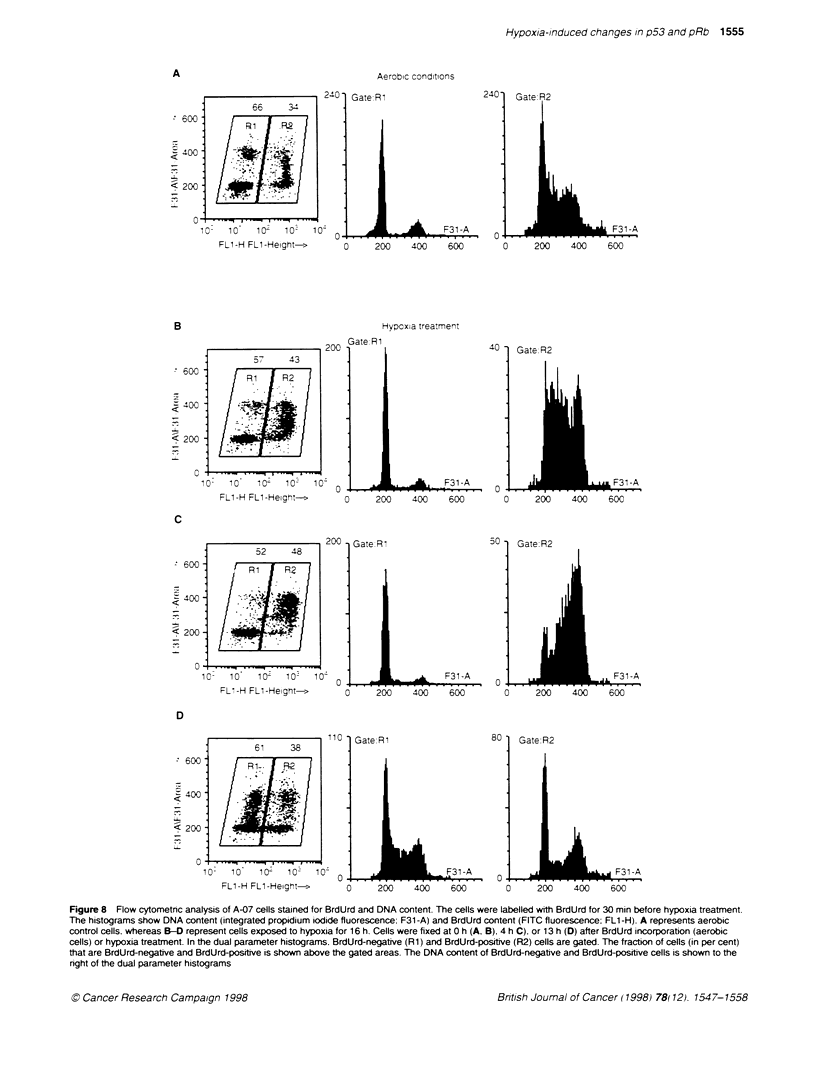
- Amellem O., Stokke T., Sandvik J. A., Smedshammer L., Pettersen E. O. Hypoxia-induced apoptosis in human cells with normal p53 status and function, without any alteration in the nuclear protein level. Exp Cell Res. 1997 May 1;232(2):361–370. doi: 10.1006/excr.1997.3497. [DOI] [PubMed] [Google Scholar]
- Bedford J. S., Mitchell J. B. The effect of hypoxia on the growth and radiation response of mammalian cells in culture. Br J Radiol. 1974 Oct;47(562):687–696. doi: 10.1259/0007-1285-47-562-687. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Giaccia A. J. Tumour hypoxia: the picture has changed in the 1990s. Int J Radiat Biol. 1994 Jan;65(1):95–102. doi: 10.1080/09553009414550131. [DOI] [PubMed] [Google Scholar]
- Demers G. W., Foster S. A., Halbert C. L., Galloway D. A. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4382–4386. doi: 10.1073/pnas.91.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers L. M., Kleerekoper M. Recent advances in biochemical markers of bone turnover. Clin Chem. 1994 Nov;40(11 Pt 1):1994–1995. [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Graeber T. G., Osmanian C., Jacks T., Housman D. E., Koch C. J., Lowe S. W., Giaccia A. J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996 Jan 4;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Graeber T. G., Peterson J. F., Tsai M., Monica K., Fornace A. J., Jr, Giaccia A. J. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994 Sep;14(9):6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapajärvi T., Kivinen L., Pitkänen K., Laiho M. Cell cycle dependent effects of u.v.-radiation on p53 expression and retinoblastoma protein phosphorylation. Oncogene. 1995 Jul 6;11(1):151–159. [PubMed] [Google Scholar]
- Hall E. J., Bedford J. S., Oliver R. Extreme hypoxia; its effect on the survival of mammalian cells irradiated at high and low dose-rates. Br J Radiol. 1966 Apr;39(460):302–307. doi: 10.1259/0007-1285-39-460-302. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Rowan S., Oren M. p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene. 1995 Apr 20;10(8):1563–1571. [PubMed] [Google Scholar]
- Hickman E. S., Picksley S. M., Vousden K. H. Cells expressing HPV16 E7 continue cell cycle progression following DNA damage induced p53 activation. Oncogene. 1994 Aug;9(8):2177–2181. [PubMed] [Google Scholar]
- Hill R. P. Tumor progression: potential role of unstable genomic changes. Cancer Metastasis Rev. 1990 Sep;9(2):137–147. doi: 10.1007/BF00046340. [DOI] [PubMed] [Google Scholar]
- Höckel M., Schlenger K., Knoop C., Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991 Nov 15;51(22):6098–6102. [PubMed] [Google Scholar]
- Ko L. J., Prives C. p53: puzzle and paradigm. Genes Dev. 1996 May 1;10(9):1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- Komarova E. A., Zelnick C. R., Chin D., Zeremski M., Gleiberman A. S., Bacus S. S., Gudkov A. V. Intracellular localization of p53 tumor suppressor protein in gamma-irradiated cells is cell cycle regulated and determined by the nucleus. Cancer Res. 1997 Dec 1;57(23):5217–5220. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Howell R. L., Smith H. C. Hypoxic stress induces reversible hypophosphorylation of pRB and reduction in cyclin A abundance independent of cell cycle progression. Oncogene. 1993 Feb;8(2):331–339. [PubMed] [Google Scholar]
- Luk C. K., Veinot-Drebot L., Tjan E., Tannock I. F. Effect of transient hypoxia on sensitivity to doxorubicin in human and murine cell lines. J Natl Cancer Inst. 1990 Apr 18;82(8):684–692. doi: 10.1093/jnci/82.8.684. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Bernhard E. J., Garza L., McKenna W. G., Koch C. J. Induction of apoptosis at different oxygen tensions: evidence that oxygen radicals do not mediate apoptotic signaling. Cancer Res. 1995 Mar 1;55(5):995–998. [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Overgaard J., Hansen H. S., Jørgensen K., Hjelm Hansen M. Primary radiotherapy of larynx and pharynx carcinoma--an analysis of some factors influencing local control and survival. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):515–521. doi: 10.1016/0360-3016(86)90058-1. [DOI] [PubMed] [Google Scholar]
- Pettersen E. O., Juul N. O., Rønning O. W. Regulation of protein metabolism of human cells during and after acute hypoxia. Cancer Res. 1986 Sep;46(9):4346–4351. [PubMed] [Google Scholar]
- Rofstad E. K., Eide K., Skøyum R., Hystad M. E., Lyng H. Apoptosis, energy metabolism, and fraction of radiobiologically hypoxic cells: a study of human melanoma multicellular spheroids. Int J Radiat Biol. 1996 Sep;70(3):241–249. doi: 10.1080/095530096144978. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Orthotopic human melanoma xenograft model systems for studies of tumour angiogenesis, pathophysiology, treatment sensitivity and metastatic pattern. Br J Cancer. 1994 Nov;70(5):804–812. doi: 10.1038/bjc.1994.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad E. K. Retention of cellular radiation sensitivity in cell and xenograft lines established from human melanoma surgical specimens. Cancer Res. 1992 Apr 1;52(7):1764–1769. [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Sanna K., Rofstad E. K. Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer. 1994 Jul 15;58(2):258–262. doi: 10.1002/ijc.2910580219. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kamiike W., Itoh Y., Hasegawa J., Yamabe K., Otsuki Y., Matsuda H., Tsujimoto Y. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996 May 1;56(9):2161–2166. [PubMed] [Google Scholar]
- Shrieve D. C., Deen D. F., Harris J. W. Effects of extreme hypoxia on the growth and viability of EMT6/SF mouse tumor cells in vitro. Cancer Res. 1983 Aug;43(8):3521–3527. [PubMed] [Google Scholar]
- Slebos R. J., Lee M. H., Plunkett B. S., Kessis T. D., Williams B. O., Jacks T., Hedrick L., Kastan M. B., Cho K. R. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro I. J., Rice G. C., Durand R. E., Stickler R., Ling C. C. Cell killing, radiosensitization and cell cycle redistribution induced by chronic hypoxia. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1275–1280. doi: 10.1016/0360-3016(84)90332-8. [DOI] [PubMed] [Google Scholar]
- Stokke T., Erikstein B. K., Smedshammer L., Boye E., Steen H. B. The retinoblastoma gene product is bound in the nucleus in early G1 phase. Exp Cell Res. 1993 Jan;204(1):147–155. doi: 10.1006/excr.1993.1019. [DOI] [PubMed] [Google Scholar]
- Sugano T., Nitta M., Ohmori H., Yamaizumi M. Nuclear accumulation of p53 in normal human fibroblasts is induced by various cellular stresses which evoke the heat shock response, independently of the cell cycle. Jpn J Cancer Res. 1995 May;86(5):415–418. doi: 10.1111/j.1349-7006.1995.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D. J. Nuclear binding of purified retinoblastoma gene product is determined by cell cycle-regulated phosphorylation. Mol Cell Biol. 1992 Feb;12(2):435–443. doi: 10.1128/mcb.12.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Yao K. S., Clayton M., O'Dwyer P. J. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J Natl Cancer Inst. 1995 Jan 18;87(2):117–122. doi: 10.1093/jnci/87.2.117. [DOI] [PubMed] [Google Scholar]




