Abstract
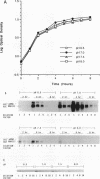
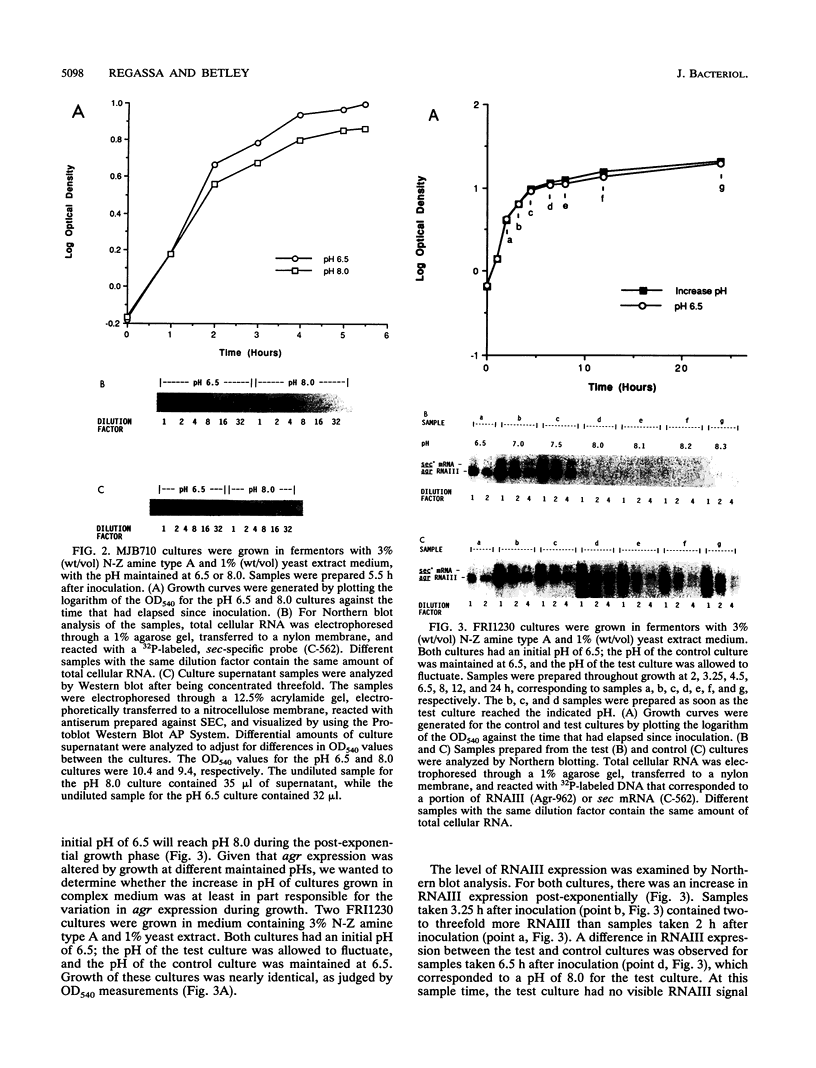
The effect of alkaline pH on expression of the accessory gene regulator (agr) in Staphylococcus aureus was examined. agr, a global regulator, affects the expression of numerous exoproteins, including alpha-hemolysin, toxic shock syndrome toxin 1, protein A, and staphylococcal enterotoxins types B, C, and D. agr contains two major, divergent transcripts, designated RNAII and RNAIII. In this study, the level of RNAIII was used to monitor agr expression because this transcript and/or its protein product(s) appears to be responsible for altering target gene expression. S. aureus FRI1230 and its Agr- derivative were examined in a fermentor system which allowed batch cultures to be maintained at a constant pH. FRI1230 cultures were grown at pH 6.5, 7.0, 7.5, and 8.0. Northern (RNA blot) analysis of samples revealed that maximal agr expression occurred at pH 7.0, with virtually no RNAIII observed at pH 8.0. The effect of alkaline pH on an agr target gene, sec, was also evaluated. sec expression was reduced at alkaline pH in strain FRI1230 (Agr+) but not in its Agr- derivative, indicating that an intact agr allele is required for the pH effect on sec. Examination of batch cultures under conditions of nonmaintained pH gave results that were also consistent with a role for alkaline pH in repressing agr expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. F., Silverman G. J. Staphylococcal enterotoxin B and nuclease production under controlled dissolved oxygen conditions. Appl Microbiol. 1974 Oct;28(4):628–637. doi: 10.1128/am.28.4.628-637.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone-Post P., Malyankar U., Khan S. A. Role of host factors in the regulation of the enterotoxin B gene. J Bacteriol. 1991 Mar;173(5):1827–1830. doi: 10.1128/jb.173.5.1827-1830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. II. Glucose repression of toxin formation. Infect Immun. 1972 Nov;6(5):689–694. doi: 10.1128/iai.6.5.689-694.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill M. E., Khan S. A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988 May 5;263(13):6276–6280. [PubMed] [Google Scholar]
- Janzon L., Arvidson S. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990 May;9(5):1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Löfdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989 Nov;219(3):480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Mallonee D. H., Glatz B. A., Pattee P. A. Chromosomal mapping of a gene affecting enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1982 Feb;43(2):397–402. doi: 10.1128/aem.43.2.397-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Collins W. S., 2nd, McGann V. Staphylococcus aureus enterotoxin B release (excretion) under controlled conditions of fermentation. Appl Microbiol. 1973 May;25(5):770–773. doi: 10.1128/am.25.5.770-773.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeldt E., Janzon L., Arvidson S., Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988 Mar;211(3):435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Regassa L. B., Couch J. L., Betley M. J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991 Mar;59(3):955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Novick R. P., Betley M. J. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect Immun. 1992 Aug;60(8):3381–3388. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Noleto A. L., Khoe G. P., Bergdoll M. S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984 Sep;45(3):625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R. F., Weiss K. F. Production of staphylococcal enterotoxins A, B, and C in various media. Appl Microbiol. 1969 Dec;18(6):1041–1043. doi: 10.1128/am.18.6.1041-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler P., Weisblum B. Erythromycin-induced stabilization of ermA messenger RNA in Staphylococcus aureus and Bacillus subtilis. J Mol Biol. 1988 Oct 20;203(4):905–915. doi: 10.1016/0022-2836(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Sarafian S. K., Morse S. A. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J Med Microbiol. 1987 Aug;24(1):75–81. doi: 10.1099/00222615-24-1-75. [DOI] [PubMed] [Google Scholar]
- Schroeder C. J., Pattee P. A. Transduction analysis of transposon Tn551 insertions in the trp-thy region of the Staphylococcus aureus chromosome. J Bacteriol. 1984 Feb;157(2):533–537. doi: 10.1128/jb.157.2.533-537.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F., Kornblum J., Novick R. P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991 Oct;173(20):6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]