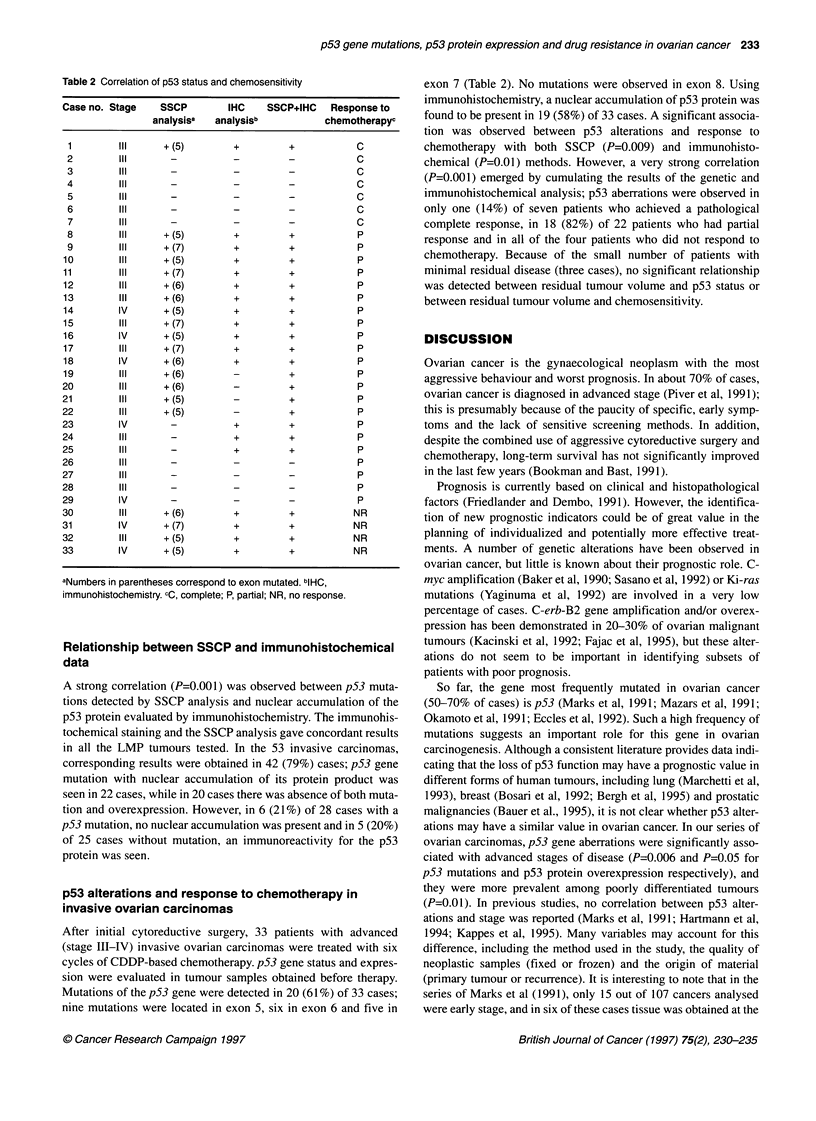
Abstract
Chemotherapeutic management of ovarian cancers is a difficult task as these neoplasms show significant differences in chemosensitivity, even if they share identical clinicopathological features. The present study was undertaken to investigate the prognostic and predictive role of p53 alterations in ovarian cancer. To this end, using different technical approaches, i.e. genetic and immunohistochemical analyses, we analysed a series of 68 ovarian neoplasms including 15 low malignant potential (LMP) tumours and 53 invasive carcinomas. We never observed p53 abnormalities in LMP tumours. p53 alterations were present only in invasive ovarian carcinomas, and they were detected much more frequently in tumours characterized by high histological grade (P = 0.01) and advanced-stage disease (P = 0.006 and P = 0.05 for gene mutations and protein expression respectively). For 33 patients with invasive ovarian cancer, information was available concerning response to cisplatin-based chemotherapy. A strong correlation (P = 0.001) has emerged between p53 alterations and response to chemotherapy; only one (14%) of seven patients who had a pathological complete response to antiblastic drugs showed p53 aberrations, whereas 18 (82%) of 22 cases with partial response and all of the four non-responsive patients scored positive for p53 abnormalities. We also observed that patients with p53 mutations had a significantly shorter progression-free survival than patients with p53-negative tumours (P = 0.05). Taken together, our results strongly suggest that in epithelial ovarian malignancies tumours showing p53 aberrations are significantly less sensitive to chemotherapy and more aggressive than those with functional p53. Thus, a routine analysis of this gene could have profound implications for the treatment of ovarian cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker V. V., Borst M. P., Dixon D., Hatch K. D., Shingleton H. M., Miller D. c-myc amplification in ovarian cancer. Gynecol Oncol. 1990 Sep;38(3):340–342. doi: 10.1016/0090-8258(90)90069-w. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bookman M. A., Bast R. C., Jr The immunobiology and immunotherapy of ovarian cancer. Semin Oncol. 1991 Jun;18(3):270–291. [PubMed] [Google Scholar]
- Bosari S., Lee A. K., Viale G., Heatley G. J., Coggi G. Abnormal p53 immunoreactivity and prognosis in node-negative breast carcinomas with long-term follow-up. Virchows Arch A Pathol Anat Histopathol. 1992;421(4):291–295. doi: 10.1007/BF01660975. [DOI] [PubMed] [Google Scholar]
- Bosari S., Viale G., Radaelli U., Bossi P., Bonoldi E., Coggi G. p53 accumulation in ovarian carcinomas and its prognostic implications. Hum Pathol. 1993 Nov;24(11):1175–1179. doi: 10.1016/0046-8177(93)90212-y. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990 Aug-Sep;2(8-9):275–280. [PubMed] [Google Scholar]
- Eccles D. M., Brett L., Lessells A., Gruber L., Lane D., Steel C. M., Leonard R. C. Overexpression of the p53 protein and allele loss at 17p13 in ovarian carcinoma. Br J Cancer. 1992 Jan;65(1):40–44. doi: 10.1038/bjc.1992.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajac A., Benard J., Lhomme C., Rey A., Duvillard P., Rochard F., Bernaudin J. F., Riou G. c-erbB2 gene amplification and protein expression in ovarian epithelial tumors: evaluation of their respective prognostic significance by multivariate analysis. Int J Cancer. 1995 Apr 21;64(2):146–151. doi: 10.1002/ijc.2910640213. [DOI] [PubMed] [Google Scholar]
- Fisher D. E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994 Aug 26;78(4):539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Dembo A. J. Prognostic factors in ovarian cancer. Semin Oncol. 1991 Jun;18(3):205–212. [PubMed] [Google Scholar]
- Gately D. P., Howell S. B. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993 Jun;67(6):1171–1176. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann L. C., Podratz K. C., Keeney G. L., Kamel N. A., Edmonson J. H., Grill J. P., Su J. Q., Katzmann J. A., Roche P. C. Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol. 1994 Jan;12(1):64–69. doi: 10.1200/JCO.1994.12.1.64. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Mayer A. G., King B. L., Carter D., Chambers S. K. NEU protein overexpression in benign, borderline, and malignant ovarian neoplasms. Gynecol Oncol. 1992 Mar;44(3):245–253. doi: 10.1016/0090-8258(92)90051-j. [DOI] [PubMed] [Google Scholar]
- Kappes S., Milde-Langosch K., Kressin P., Passlack B., Dockhorn-Dworniczak B., Röhlke P., Löning T. p53 mutations in ovarian tumors, detected by temperature-gradient gel electrophoresis, direct sequencing and immunohistochemistry. Int J Cancer. 1995 Feb 20;64(1):52–59. doi: 10.1002/ijc.2910640111. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Koechli O., Schaer G. N., Seifert B., Hornung R., Haller U., Eppenberger U., Mueller H. Mutant p53 protein associated with chemosensitivity in breast cancer specimens. Lancet. 1994 Dec 10;344(8937):1647–1648. doi: 10.1016/s0140-6736(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Kohler M. F., Marks J. R., Wiseman R. W., Jacobs I. J., Davidoff A. M., Clarke-Pearson D. L., Soper J. T., Bast R. C., Jr, Berchuck A. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993 Sep 15;85(18):1513–1519. doi: 10.1093/jnci/85.18.1513. [DOI] [PubMed] [Google Scholar]
- Levesque M. A., Katsaros D., Yu H., Zola P., Sismondi P., Giardina G., Diamandis E. P. Mutant p53 protein overexpression is associated with poor outcome in patients with well or moderately differentiated ovarian carcinoma. Cancer. 1995 Mar 15;75(6):1327–1338. doi: 10.1002/1097-0142(19950315)75:6<1327::aid-cncr2820750615>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Marchetti A., Buttitta F., Merlo G., Diella F., Pellegrini S., Pepe S., Macchiarini P., Chella A., Angeletti C. A., Callahan R. p53 alterations in non-small cell lung cancers correlate with metastatic involvement of hilar and mediastinal lymph nodes. Cancer Res. 1993 Jun 15;53(12):2846–2851. [PubMed] [Google Scholar]
- Marks J. R., Davidoff A. M., Kerns B. J., Humphrey P. A., Pence J. C., Dodge R. K., Clarke-Pearson D. L., Iglehart J. D., Bast R. C., Jr, Berchuck A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991 Jun 1;51(11):2979–2984. [PubMed] [Google Scholar]
- Mazars R., Pujol P., Maudelonde T., Jeanteur P., Theillet C. p53 mutations in ovarian cancer: a late event? Oncogene. 1991 Sep;6(9):1685–1690. [PubMed] [Google Scholar]
- Milner B. J., Allan L. A., Eccles D. M., Kitchener H. C., Leonard R. C., Kelly K. F., Parkin D. E., Haites N. E. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res. 1993 May 1;53(9):2128–2132. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Nabeya Y., Loganzo F., Jr, Maslak P., Lai L., de Oliveira A. R., Schwartz G. K., Blundell M. L., Altorki N. K., Kelsen D. P., Albino A. P. The mutational status of p53 protein in gastric and esophageal adenocarcinoma cell lines predicts sensitivity to chemotherapeutic agents. Int J Cancer. 1995 Feb 20;64(1):37–46. doi: 10.1002/ijc.2910640109. [DOI] [PubMed] [Google Scholar]
- Niwa K., Itoh M., Murase T., Morishita S., Itoh N., Mori H., Tamaya T. Alteration of p53 gene in ovarian carcinoma: clinicopathological correlation and prognostic significance. Br J Cancer. 1994 Dec;70(6):1191–1197. doi: 10.1038/bjc.1994.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Okamoto A., Sameshima Y., Yokoyama S., Terashima Y., Sugimura T., Terada M., Yokota J. Frequent allelic losses and mutations of the p53 gene in human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5171–5176. [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., Orr R. M., Peacock J. H. The role of apoptosis in cell killing by cisplatin: a flow cytometric study. Br J Cancer. 1994 Jan;69(1):93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez R. P., Hamilton T. C., Ozols R. F., Young R. C. Mechanisms and modulation of resistance to chemotherapy in ovarian cancer. Cancer. 1993 Feb 15;71(4 Suppl):1571–1580. doi: 10.1002/cncr.2820710424. [DOI] [PubMed] [Google Scholar]
- Piver M. S., Baker T. R., Piedmonte M., Sandecki A. M. Epidemiology and etiology of ovarian cancer. Semin Oncol. 1991 Jun;18(3):177–185. [PubMed] [Google Scholar]
- Righetti S. C., Della Torre G., Pilotti S., Ménard S., Ottone F., Colnaghi M. I., Pierotti M. A., Lavarino C., Cornarotti M., Oriana S. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996 Feb 15;56(4):689–693. [PubMed] [Google Scholar]
- Sasano H., Nagura H., Silverberg S. G. Immunolocalization of c-myc oncoprotein in mucinous and serous adenocarcinomas of the ovary. Hum Pathol. 1992 May;23(5):491–495. doi: 10.1016/0046-8177(92)90125-m. [DOI] [PubMed] [Google Scholar]
- Spinardi L., Mazars R., Theillet C. Protocols for an improved detection of point mutations by SSCP. Nucleic Acids Res. 1991 Jul 25;19(14):4009–4009. doi: 10.1093/nar/19.14.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]




