Abstract
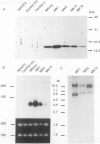
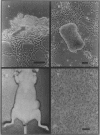
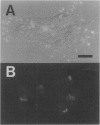
Midkine (MK) is a heparin-binding growth factor and is frequently expressed at high levels in many human carcinomas. To investigate further the roles of MK in the regulation of cell growth, we introduced MK expression in NIH3T3 cells. A mixture of transfectants of an MK expression vector, but not a control vector, formed colonies in soft agar, showed an elevated cell number at confluence, and formed tumours in nude mice. An interesting characteristic of the transformed cells was that they became spontaneously detached from the culture dish substratum. In the transformed cells, MK was not only secreted, but also localized, in the perinuclear region as spots. The present data indicate that MK has the potential to transform NIH3T3 cells and suggest that overexpression of the MK gene may promote unregulated cell growth in vivo.
Full text
PDF
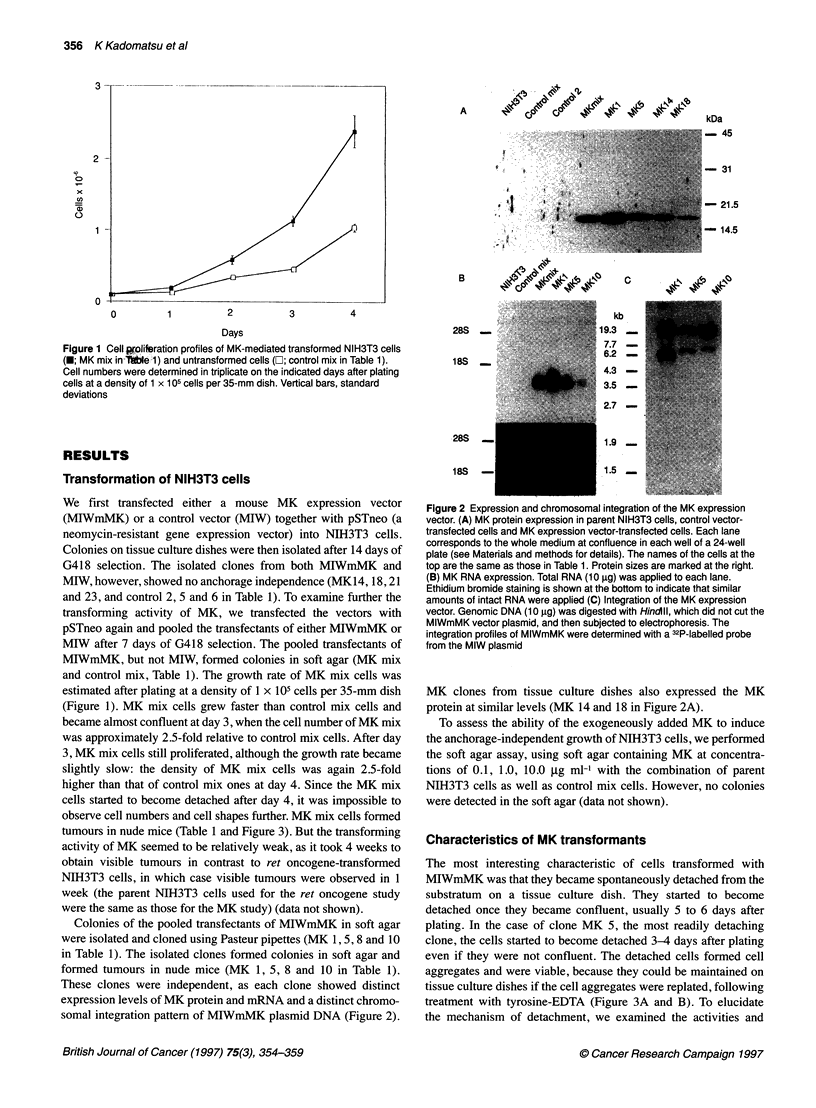
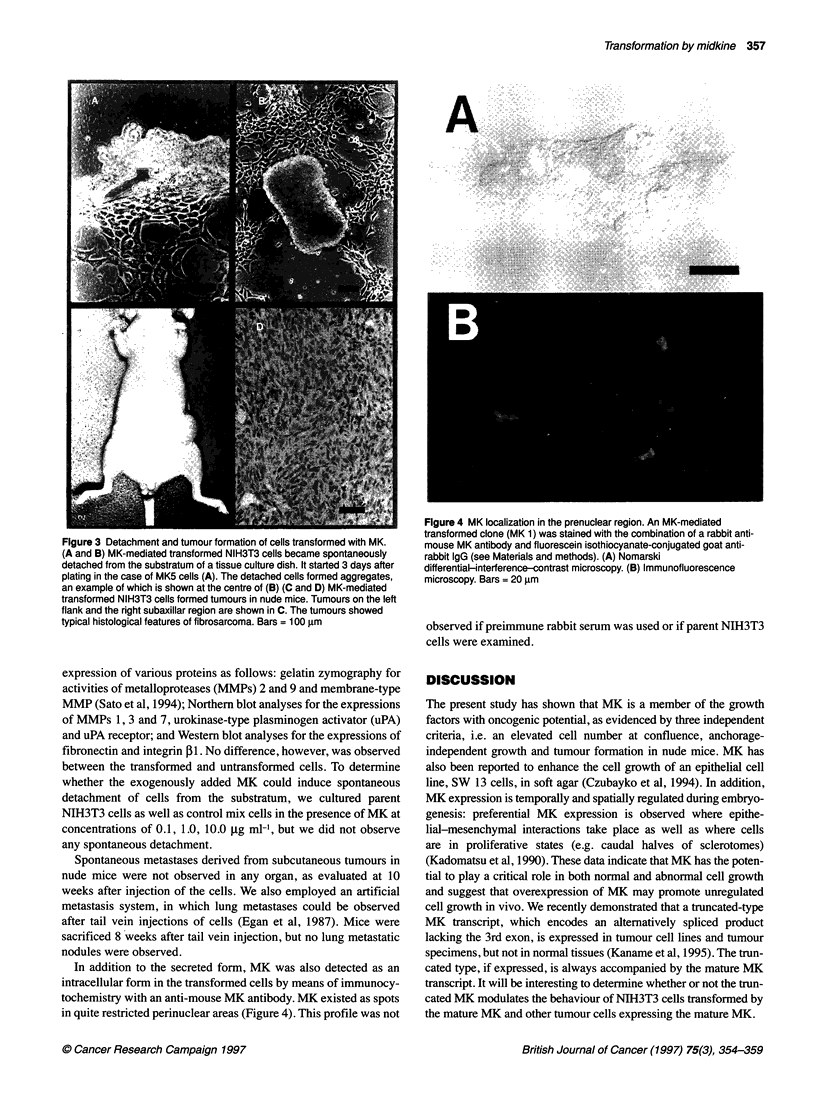


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Aridome K., Tsutsui J., Takao S., Kadomatsu K., Ozawa M., Aikou T., Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995 Jul;86(7):655–661. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai N., Iwashita T., Matsuyama M., Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995 Mar;15(3):1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. K., Li Y. S., Deuel T. F. Pleiotrophin transforms NIH 3T3 cells and induces tumors in nude mice. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):679–682. doi: 10.1073/pnas.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Czubayko F., Riegel A. T., Wellstein A. Ribozyme-targeting elucidates a direct role of pleiotrophin in tumor growth. J Biol Chem. 1994 Aug 19;269(33):21358–21363. [PubMed] [Google Scholar]
- Egan S. E., Wright J. A., Jarolim L., Yanagihara K., Bassin R. H., Greenberg A. H. Transformation by oncogenes encoding protein kinases induces the metastatic phenotype. Science. 1987 Oct 9;238(4824):202–205. doi: 10.1126/science.3659911. [DOI] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chan C. S., Milner P. G. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am J Respir Cell Mol Biol. 1993 Nov;9(5):463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- Garver R. I., Jr, Radford D. M., Donis-Keller H., Wick M. R., Milner P. G. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994 Sep 1;74(5):1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::aid-cncr2820740514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Kadomatsu K., Huang R. P., Suganuma T., Murata F., Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol. 1990 Mar;110(3):607–616. doi: 10.1083/jcb.110.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K., Tomomura M., Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1312–1318. doi: 10.1016/s0006-291x(88)80505-9. [DOI] [PubMed] [Google Scholar]
- Kaname T., Kadomatsu K., Aridome K., Yamashita S., Sakamoto K., Ogawa M., Muramatsu T., Yamamura K. The expression of truncated MK in human tumors. Biochem Biophys Res Commun. 1996 Feb 6;219(1):256–260. doi: 10.1006/bbrc.1996.0214. [DOI] [PubMed] [Google Scholar]
- Kojima S., Muramatsu H., Amanuma H., Muramatsu T. Midkine enhances fibrinolytic activity of bovine endothelial cells. J Biol Chem. 1995 Apr 21;270(16):9590–9596. doi: 10.1074/jbc.270.16.9590. [DOI] [PubMed] [Google Scholar]
- Krätzschmar J., Haendler B., Kojima S., Rifkin D. B., Schleuning W. D. Bovine urokinase-type plasminogen activator and its receptor: cloning and induction by retinoic acid. Gene. 1993 Mar 30;125(2):177–183. doi: 10.1016/0378-1119(93)90325-w. [DOI] [PubMed] [Google Scholar]
- Li Y. S., Milner P. G., Chauhan A. K., Watson M. A., Hoffman R. M., Kodner C. M., Milbrandt J., Deuel T. F. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990 Dec 21;250(4988):1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- Mailleux P., Vanderwinden J. M., Vanderhaeghen J. J. The new growth factor pleiotrophin (HB-GAM) mRNA is selectively present in the meningothelial cells of human meningiomas. Neurosci Lett. 1992 Aug 3;142(1):31–35. doi: 10.1016/0304-3940(92)90613-c. [DOI] [PubMed] [Google Scholar]
- Merenmies J., Rauvala H. Molecular cloning of the 18-kDa growth-associated protein of developing brain. J Biol Chem. 1990 Oct 5;265(28):16721–16724. [PubMed] [Google Scholar]
- Michikawa M., Kikuchi S., Muramatsu H., Muramatsu T., Kim S. U. Retinoic acid responsive gene product, midkine, has neurotrophic functions for mouse spinal cord and dorsal root ganglion neurons in culture. J Neurosci Res. 1993 Aug 1;35(5):530–539. doi: 10.1002/jnr.490350509. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T. A., Salmivirta M., Muramatsu T., Muramatsu H., Rauvala H., Lehtonen E., Jalkanen M., Thesleff I. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development. 1995 Jan;121(1):37–51. doi: 10.1242/dev.121.1.37. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu H., Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991 Jun 14;177(2):652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu H., Shirahama H., Yonezawa S., Maruta H., Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993 Oct;159(2):392–402. doi: 10.1006/dbio.1993.1250. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine (MK), the product of a retinoic acid responsive gene, and pleiotrophin constitute a new protein family regulating growth and differentiation. Int J Dev Biol. 1993 Mar;37(1):183–188. [PubMed] [Google Scholar]
- Nakagawara A., Milbrandt J., Muramatsu T., Deuel T. F., Zhao H., Cnaan A., Brodeur G. M. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res. 1995 Apr 15;55(8):1792–1797. [PubMed] [Google Scholar]
- Nurcombe V., Fraser N., Herlaar E., Heath J. K. MK: a pluripotential embryonic stem-cell-derived neuroregulatory factor. Development. 1992 Dec;116(4):1175–1183. doi: 10.1242/dev.116.4.1175. [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Klos A. M., Hirohashi S., Hakomori S. Human tissue fibronectin: expression of different isotypes in the adult and fetal tissues. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1012–1017. doi: 10.1016/s0006-291x(86)80145-0. [DOI] [PubMed] [Google Scholar]
- Take M., Tsutsui J., Obama H., Ozawa M., Nakayama T., Maruyama I., Arima T., Muramatsu T. Identification of nucleolin as a binding protein for midkine (MK) and heparin-binding growth associated molecule (HB-GAM). J Biochem. 1994 Nov;116(5):1063–1068. doi: 10.1093/oxfordjournals.jbchem.a124628. [DOI] [PubMed] [Google Scholar]
- Takeda A., Onodera H., Sugimoto A., Itoyama Y., Kogure K., Rauvala H., Shibahara S. Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience. 1995 Sep;68(1):57–64. doi: 10.1016/0306-4522(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Tomomura M., Kadomatsu K., Matsubara S., Muramatsu T. A retinoic acid-responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem. 1990 Jun 25;265(18):10765–10770. [PubMed] [Google Scholar]
- Tomomura M., Kadomatsu K., Nakamoto M., Muramatsu H., Kondoh H., Imagawa K., Muramatsu T. A retinoic acid responsive gene, MK, produces a secreted protein with heparin binding activity. Biochem Biophys Res Commun. 1990 Sep 14;171(2):603–609. doi: 10.1016/0006-291x(90)91189-y. [DOI] [PubMed] [Google Scholar]
- Tsutsui J., Kadomatsu K., Matsubara S., Nakagawara A., Hamanoue M., Takao S., Shimazu H., Ohi Y., Muramatsu T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms' tumor and other human carcinomas. Cancer Res. 1993 Mar 15;53(6):1281–1285. [PubMed] [Google Scholar]
- Unoki K., LaVail M. M. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):907–915. [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Goto M., Tsutsui J., Ozawa M., Sato E., Osame M., Muramatsu T. Midkine is present in the early stage of cerebral infarct. Brain Res Dev Brain Res. 1995 Mar 16;85(1):25–30. doi: 10.1016/0165-3806(94)00183-z. [DOI] [PubMed] [Google Scholar]