Abstract
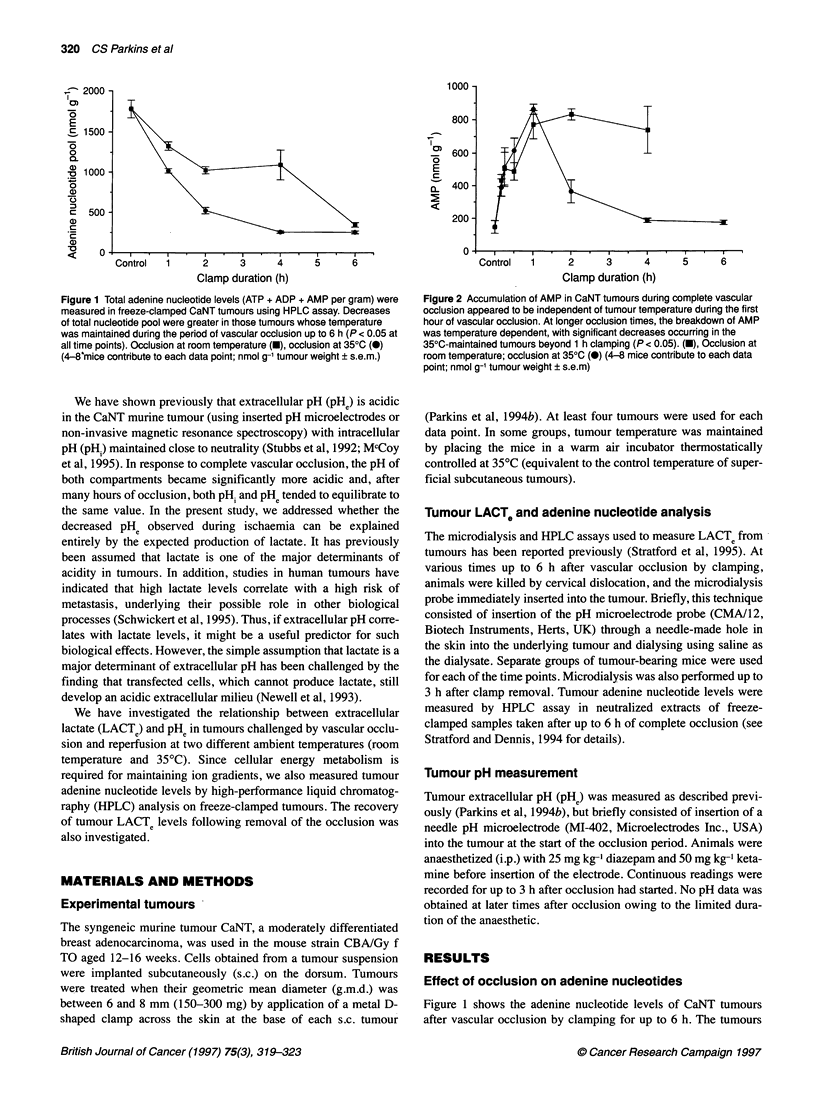
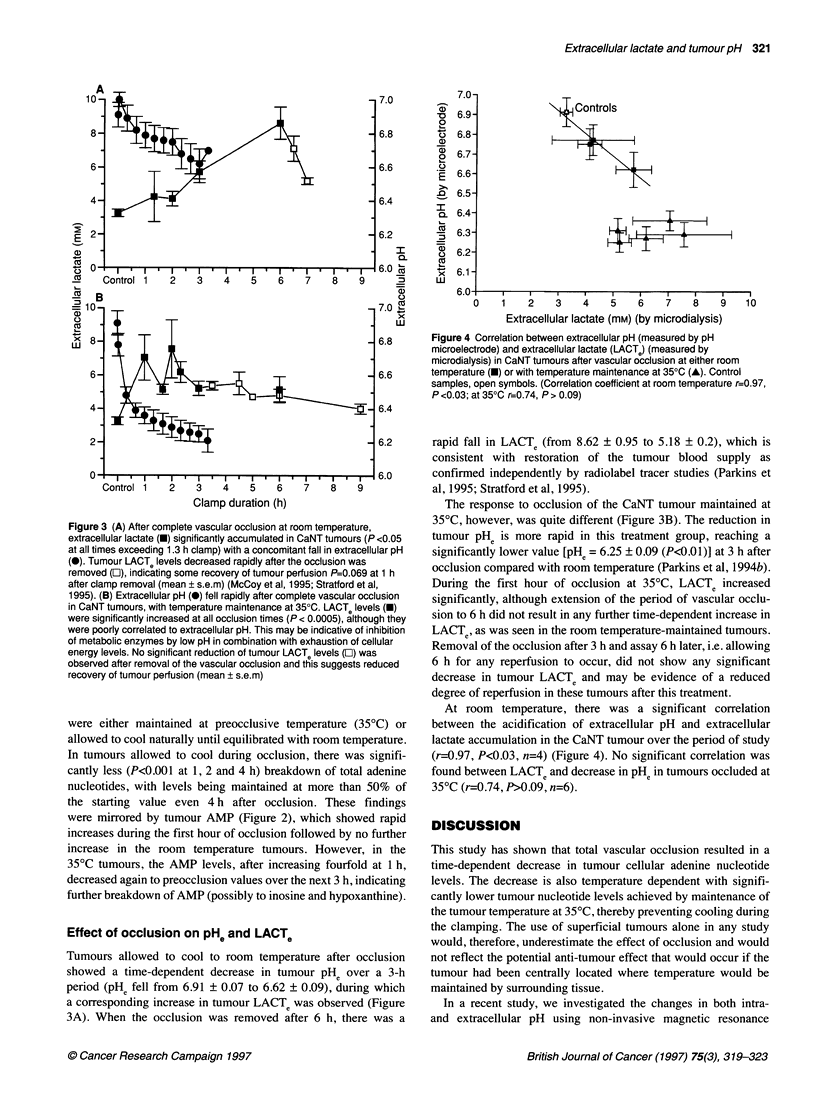
We have studied the relationship between extracellular lactate (LACTe) and extracellular pH (pHe) in murine tumours after vascular occlusion (clamping) followed by reperfusion. In tumours occluded at ambient room temperature, LACTe, measured by microdialysis, increased linearly with time and correlated strongly with the acidification of the extracellular compartment (r=0.97, P<0.03, n=4). Significant decrease in LACTe was evident following removal of occlusion at room temperature and is consistent with vascular reperfusion. Occlusion at 35 degrees C, i.e. to maintain tumour temperature during occlusion, resulted in an initial increase in LACTe, which mirrored a rapid reduction in pHe. However further reductions in pHe occurred without increase in LACTe. During vascular occlusion, tumour adenine nucleotide pool decreased and AMP accumulated. AMP subsequently decreased in the 35 degrees C group and this may contribute to the observed differences in accumulation of LACTe, and capacity to recover from vascular occlusion, between the two treatment groups. These data show that extracellular lactate concentration is a good predictor for tumour pH when adequate energy sources are available within the tumour. However, under conditions of more severe stress, resulting in abolition of primary energy stores and cell death, the pHe continues to decline in the absence of a corresponding accumulation of extracellular lactate. This emphasizes the fact that other processes, apart from lactate production, can contribute to reduction in extracellular pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Connors H. P., Relman A. S., Karnovsky M. L. Factors that control the effect of pH on glycolysis in leukocytes. J Biol Chem. 1969 Jan 25;244(2):384–390. [PubMed] [Google Scholar]
- McCoy C. L., Parkins C. S., Chaplin D. J., Griffiths J. R., Rodrigues L. M., Stubbs M. The effect of blood flow modification on intra- and extracellular pH measured by 31P magnetic resonance spectroscopy in murine tumours. Br J Cancer. 1995 Oct;72(4):905–911. doi: 10.1038/bjc.1995.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K., Franchi A., Pouysségur J., Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins C. S., Chadwick J. A., Chaplin D. J. Enhancement of chlorambucil cytotoxicity by combination with flavone acetic acid in a murine tumour. Anticancer Res. 1994 Jul-Aug;14(4A):1603–1608. [PubMed] [Google Scholar]
- Parkins C. S., Dennis M. F., Stratford M. R., Hill S. A., Chaplin D. J. Ischemia reperfusion injury in tumors: the role of oxygen radicals and nitric oxide. Cancer Res. 1995 Dec 15;55(24):6026–6029. [PubMed] [Google Scholar]
- Parkins C. S., Hill S. A., Lonergan S. J., Horsman M. R., Chadwick J. A., Chaplin D. J. Ischaemia induced cell death in tumors: importance of temperature and pH. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):499–503. doi: 10.1016/0360-3016(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Rotin D., Steele-Norwood D., Grinstein S., Tannock I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 1989 Jan 1;49(1):205–211. [PubMed] [Google Scholar]
- Schwickert G., Walenta S., Sundfør K., Rofstad E. K., Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995 Nov 1;55(21):4757–4759. [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford M. R., Parkins C. S., Everett S. A., Dennis M. F., Stubbs M., Hill S. A. Analysis of the acidic microenvironment in murine tumours by high-performance ion chromatography. J Chromatogr A. 1995 Jul 7;706(1-2):459–462. doi: 10.1016/0021-9673(95)00016-g. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Bhujwalla Z. M., Tozer G. M., Rodrigues L. M., Maxwell R. J., Morgan R., Howe F. A., Griffiths J. R. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992 Nov-Dec;5(6):351–359. doi: 10.1002/nbm.1940050606. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L., Howe F. A., Wang J., Jeong K. S., Veech R. L., Griffiths J. R. Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res. 1994 Aug 1;54(15):4011–4016. [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Ui M. A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochim Biophys Acta. 1966 Aug 24;124(2):310–322. doi: 10.1016/0304-4165(66)90194-2. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Veech R. L. The metabolism of lactate. NMR Biomed. 1991 Apr;4(2):53–58. doi: 10.1002/nbm.1940040204. [DOI] [PubMed] [Google Scholar]


