Abstract
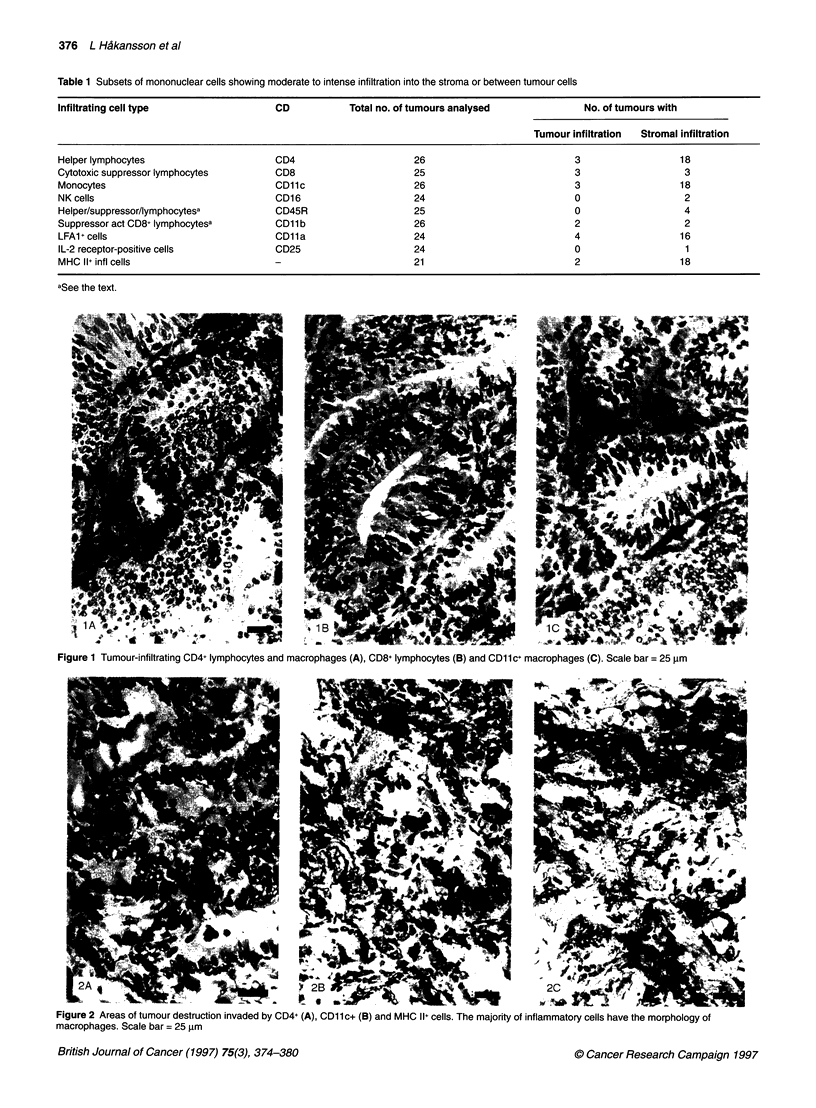
Local immunoregulation mediated by mononuclear tumour-infiltrating cells is considered of importance for tumour progression of colorectal cancer, although the balance between immunosuppressor and cytotoxic activities is unclear. Colorectal cancers from 26 patients were investigated using a panel of monoclonal antibodies in order to identify subsets of mononuclear inflammatory cells and to study their pattern of distribution in relation to tumour stage and cytotoxic immune reactivity against the tumour. In all but five tumours, mononuclear cells, lymphocytes or monocytes were present in fairly large numbers, particularly in the stroma. The infiltration of CD4+ mononuclear cells predominated over the CD8+ subset. Infiltration near the tumour cells was found in four cancers only. Stromal infiltration of CD11c+ macrophages was found in all but eight tumours. Small regressive areas, in which the histological architecture of the tumours was broken down, were found in 17 tumours with intense or moderate infiltration by CD4+ lymphocytes or CD11c+ macrophages. Probably this destruction of tumour tissue was caused by cytotoxic activity of the tumour-infiltrating mononuclear cells. In Dukes' class A and B tumours, CD4+ lymphocytes predominated over CD4+ cells with macrophage morphology, but the latter were increasingly found in Dukes' class C and D disease. The occurrence of MHC II-positive macrophages and lymphocytes in different Dukes' classes was similar to that of CD4+ cells. In contrast to this, CD11c+ and CD11a+ cells were more frequent in Dukes' A and B class tumours compared with Dukes' C and D. Four out of nine tumours of the latter stages showed a poor inflammatory reaction. The interpretation of our results is that the subsets of tumour-infiltrating mononuclear cells change with advancing Dukes' class and that the local immune control is gradually broken down in progressive tumour growth, even if some cytotoxic activity is still present.
Full text
PDF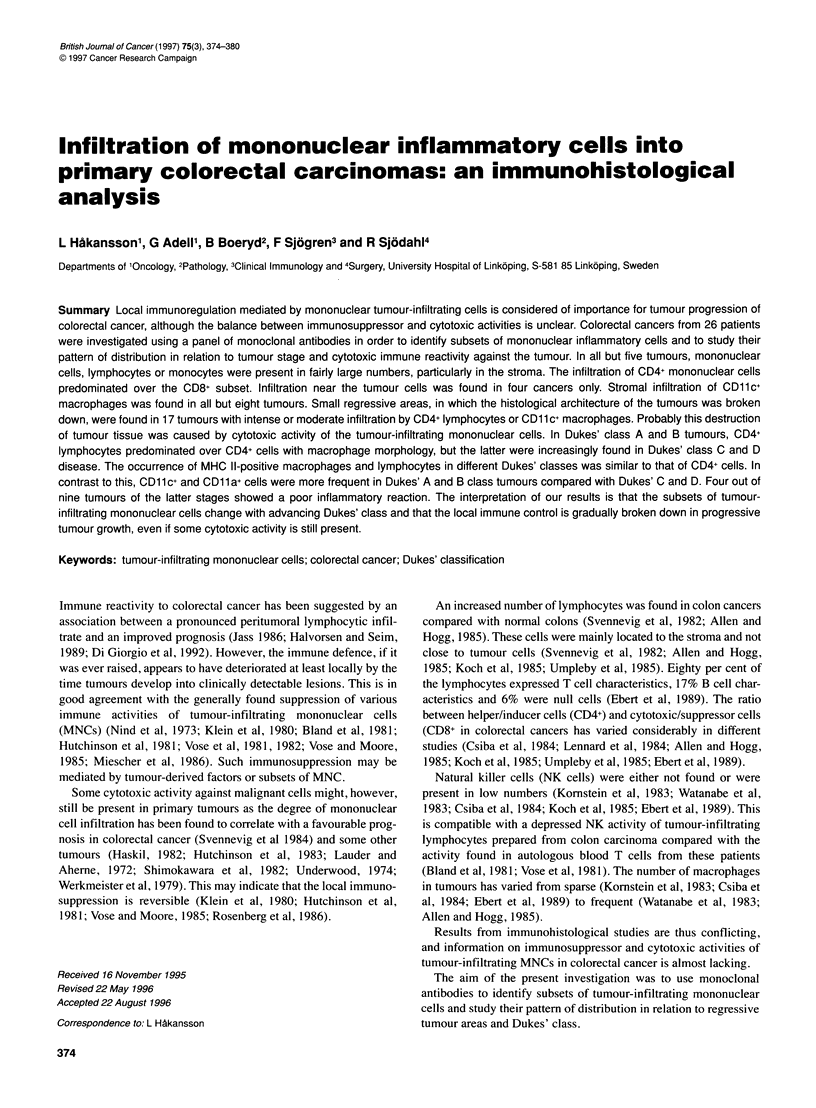





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Hogg N. Monocytes and other infiltrating cells in human colorectal tumours identified by monoclonal antibodies. Immunology. 1985 Jun;55(2):289–299. [PMC free article] [PubMed] [Google Scholar]
- Andersen S. N., Rognum T. O., Lund E., Meling G. I., Hauge S. Strong HLA-DR expression in large bowel carcinomas is associated with good prognosis. Br J Cancer. 1993 Jul;68(1):80–85. doi: 10.1038/bjc.1993.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Embleton M. J., Price M. R. Inhibition of lymphocyte cytotoxicity for human colon carcinoma by treatment with solubilized tumour membrane fractions. Int J Cancer. 1973 Jul 15;12(1):84–92. doi: 10.1002/ijc.2910120109. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Britton D. C., Richens E. R., Pledger J. V. Peripheral, mucosal, and tumour-infiltrating components of cellular immunity in cancer of the large bowel. Gut. 1981 Sep;22(9):744–751. doi: 10.1136/gut.22.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiba A., Whitwell H. L., Moore M. Distribution of histocompatibility and leucocyte differentiation antigens in normal human colon and in benign and malignant colonic neoplasms. Br J Cancer. 1984 Nov;50(5):699–709. doi: 10.1038/bjc.1984.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. The membrane antigens of human colorectal cancer cells: demonstration with monoclonal antibodies of heterogeneity within and between tumours and of anomalous expression of HLA-DR. Eur J Cancer Clin Oncol. 1983 Feb;19(2):209–220. doi: 10.1016/0277-5379(83)90419-4. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Ting A., Fabre J. W. Anomolous expression of HLA-DR antigens on human colorectal cancer cells. J Immunol. 1982 Aug;129(2):447–449. [PubMed] [Google Scholar]
- Di Giorgio A., Botti C., Tocchi A., Mingazzini P., Flammia M. The influence of tumor lymphocytic infiltration on long term survival of surgically treated colorectal cancer patients. Int Surg. 1992 Oct-Dec;77(4):256–260. [PubMed] [Google Scholar]
- Durrant L. G., Ballantyne K. C., Armitage N. C., Robins R. A., Marksman R., Hardcastle J. D., Baldwin R. W. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br J Cancer. 1987 Oct;56(4):425–432. doi: 10.1038/bjc.1987.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert E. C., Brolin R. E., Roberts A. I. Characterization of activated lymphocytes in colon cancer. Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 1):72–81. doi: 10.1016/0090-1229(89)90223-7. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Roberts A. I., O'Connell S. M., Robertson F. M., Nagase H. Characterization of an immunosuppressive factor derived from colon cancer cells. J Immunol. 1987 Apr 1;138(7):2161–2168. [PubMed] [Google Scholar]
- Halvorsen T. B., Seim E. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol. 1989 Feb;42(2):162–166. doi: 10.1136/jcp.42.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka H., Imamura M., Ishii Y., Kohama G., Kikuchi K. Immunohistologic detection of lymphocyte subpopulations infiltrating in human oral cancer with special reference to its clinical significance. Cancer. 1984 Jun 1;53(11):2456–2466. doi: 10.1002/1097-0142(19840601)53:11<2456::aid-cncr2820531116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hiratsuka H., Imamura M., Kasai K., Kamiya H., Ishii Y., Kohama G., Kikuchi K. Lymphocyte subpopulations and T-cell subsets in human oral cancer tissues: immunohistologic analysis by monoclonal antibodies. Am J Clin Pathol. 1984 Apr;81(4):464–470. doi: 10.1093/ajcp/81.4.464. [DOI] [PubMed] [Google Scholar]
- Hutchinson G. H., Heinemann D., Symes M. O., Williamson R. C. Differential immune reactivity of tumour-intrinsic and peripheral-blood lymphocytes against autoplastic colorectal carcinoma cells. Br J Cancer. 1981 Sep;44(3):396–402. doi: 10.1038/bjc.1981.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986 Jun;39(6):585–589. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Vánky F., Galili U., Vose B. M., Fopp M. Separation and characteristics of tumor-infiltrating lymphocytes in man. Contemp Top Immunobiol. 1980;10:79–107. doi: 10.1007/978-1-4684-3677-8_4. [DOI] [PubMed] [Google Scholar]
- Koch B., Giedl J., Hermanek P., Kalden J. R. The analysis of mononuclear cell infiltrations in colorectal adenocarcinoma. J Cancer Res Clin Oncol. 1985;109(2):142–151. doi: 10.1007/BF00391889. [DOI] [PubMed] [Google Scholar]
- Kornstein M. J., Brooks J. S., Elder D. E. Immunoperoxidase localization of lymphocyte subsets in the host response to melanoma and nevi. Cancer Res. 1983 Jun;43(6):2749–2753. [PubMed] [Google Scholar]
- Lauder I., Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972 Aug;26(4):321–330. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard T. W., Warford A., Taylor R. M., Shenton B. K., Proud G. In situ subpopulations of lymphocytes in human colorectal carcinomas. Invasion Metastasis. 1984;4 (Suppl 1):60–66. [PubMed] [Google Scholar]
- Miescher S., Stoeck M., Qiao L., Barras C., Barrelet L., von Fliedner V. Preferential clonogenic deficit of CD8-positive T-lymphocytes infiltrating human solid tumors. Cancer Res. 1988 Dec 15;48(24 Pt 1):6992–6998. [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Carrel S., von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986 Mar 1;136(5):1899–1907. [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nind A. P., Nairn R. C., Rolland J. M., Guli E. P., Hughes E. S. Lymphocyte anergy in patients with carcinoma. Br J Cancer. 1973 Aug;28(2):108–117. doi: 10.1038/bjc.1973.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle-Bonnet M. M., Pommier F. J., Kaplanski S., Rance R. J., Depieds R. C. Inhibition of normal allogenic lymphocyte mitogenesis by a soluble inhibitor extracted from human colonic carcinoma. J Immunol. 1976 Oct;117(4):1145–1151. [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Thorud E. Is heterogeneous expression of HLA-dr antigens and CEA along with DNA-profile variations evidence of phenotypic instability and clonal proliferation in human large bowel carcinomas? Br J Cancer. 1983 Oct;48(4):543–551. doi: 10.1038/bjc.1983.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Shimokawara I., Imamura M., Yamanaka N., Ishii Y., Kikuchi K. Identification of lymphocyte subpopulations in human breast cancer tissue and its significance: an immunoperoxidase study with anti-human T- and B-cell sera. Cancer. 1982 Apr 1;49(7):1456–1464. doi: 10.1002/1097-0142(19820401)49:7<1456::aid-cncr2820490724>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J., Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984 Mar;49(3):375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J. In situ analysis of the inflammatory cell infiltrates in colon carcinomas and in the normal colon wall. Acta Pathol Microbiol Immunol Scand A. 1982 Mar;90(2):131–137. doi: 10.1111/j.1699-0463.1982.tb00073_90a.x. [DOI] [PubMed] [Google Scholar]
- Umpleby H. C., Heinemann D., Symes M. O., Williamson R. C. Expression of histocompatibility antigens and characterization of mononuclear cell infiltrates in normal and neoplastic colorectal tissues of humans. J Natl Cancer Inst. 1985 Jun;74(6):1161–1168. [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Gallagher P., Moore M., Schofield P. F. Specific and non-specific lymphocyte cytotoxicity in colon carcinoma. Br J Cancer. 1981 Dec;44(6):846–855. doi: 10.1038/bjc.1981.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Moore M. Human tumor-infiltrating lymphocytes: a marker of host response. Semin Hematol. 1985 Jan;22(1):27–40. [PubMed] [Google Scholar]
- Watanabe S., Sato Y., Kodama T., Shimosato Y. Immunohistochemical study with monoclonal antibodies on immune response in human lung cancers. Cancer Res. 1983 Dec;43(12 Pt 1):5883–5889. [PubMed] [Google Scholar]
- Werkmeister J. A., Pihl E., Nind A. P., Flannery G. R., Nairn R. C. Immunoreactivity by intrinsic lymphoid cells in colorectal carcinoma. Br J Cancer. 1979 Dec;40(6):839–847. doi: 10.1038/bjc.1979.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead J. S., Kim Y. S. An inhibitor of lymphocyte proliferation produced by a human colonic adenocarcinoma cell line in culture. Cancer Res. 1980 Jan;40(1):29–35. [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]




