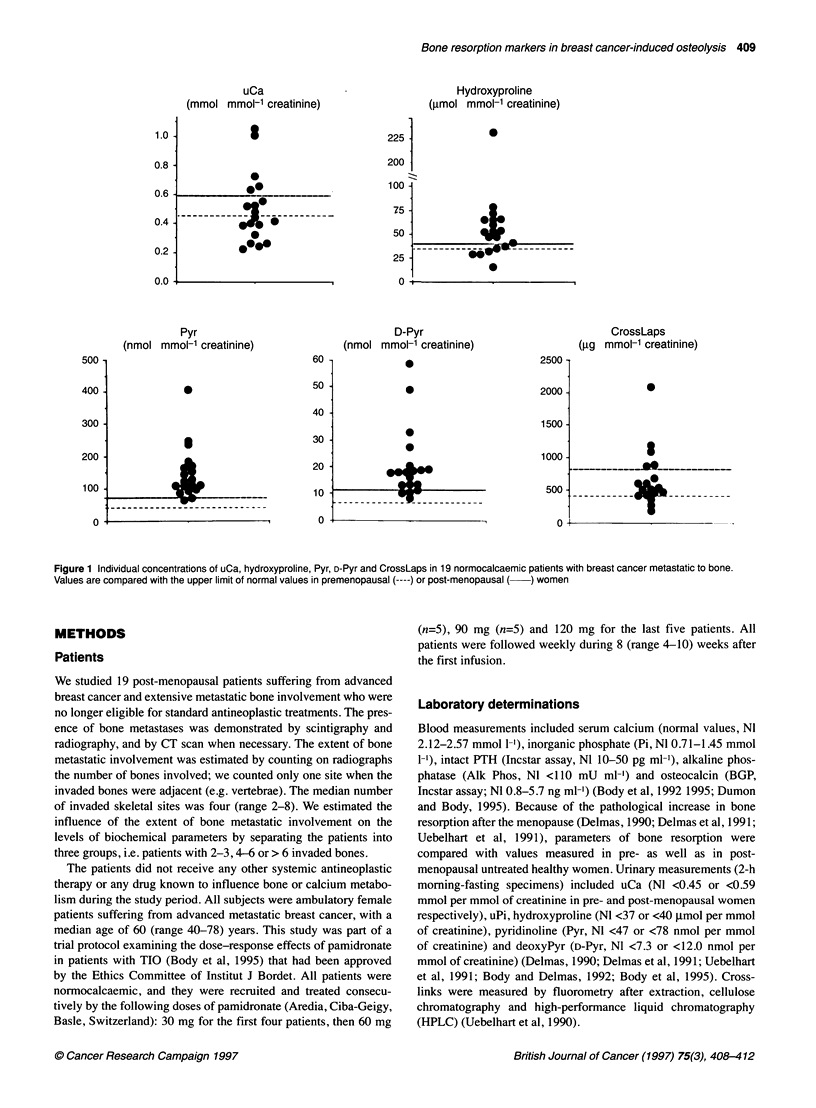
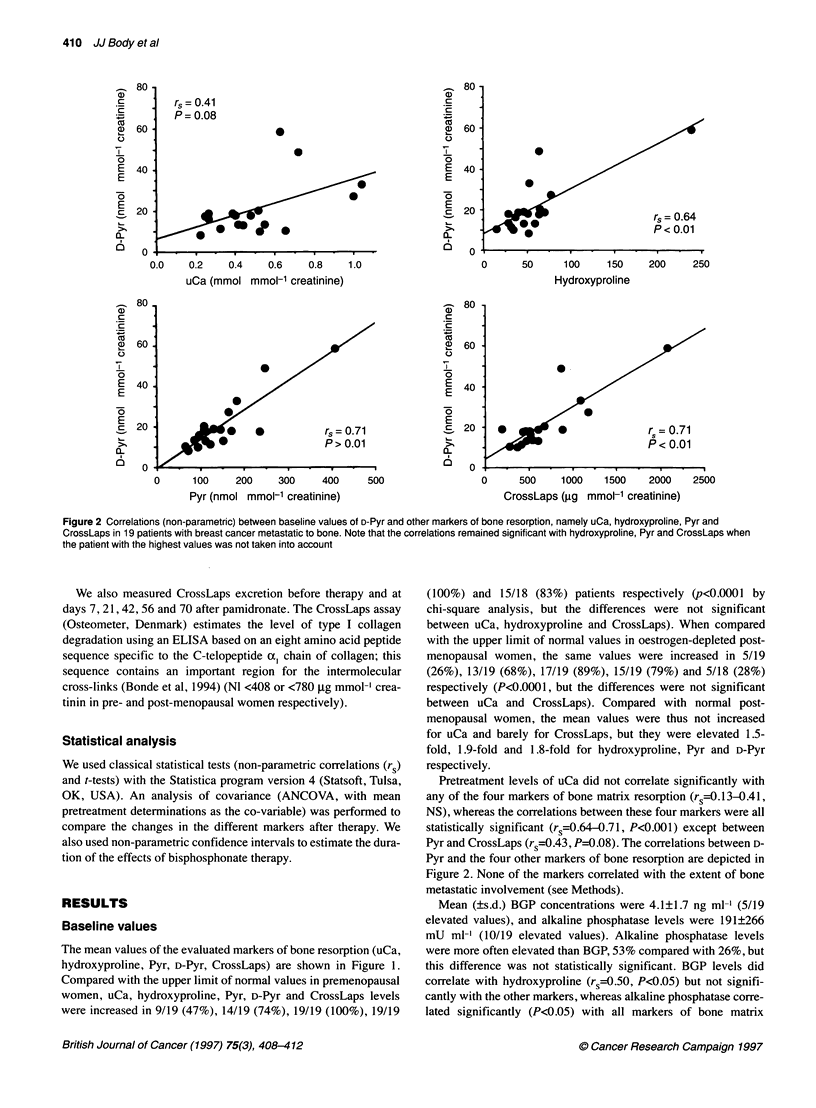
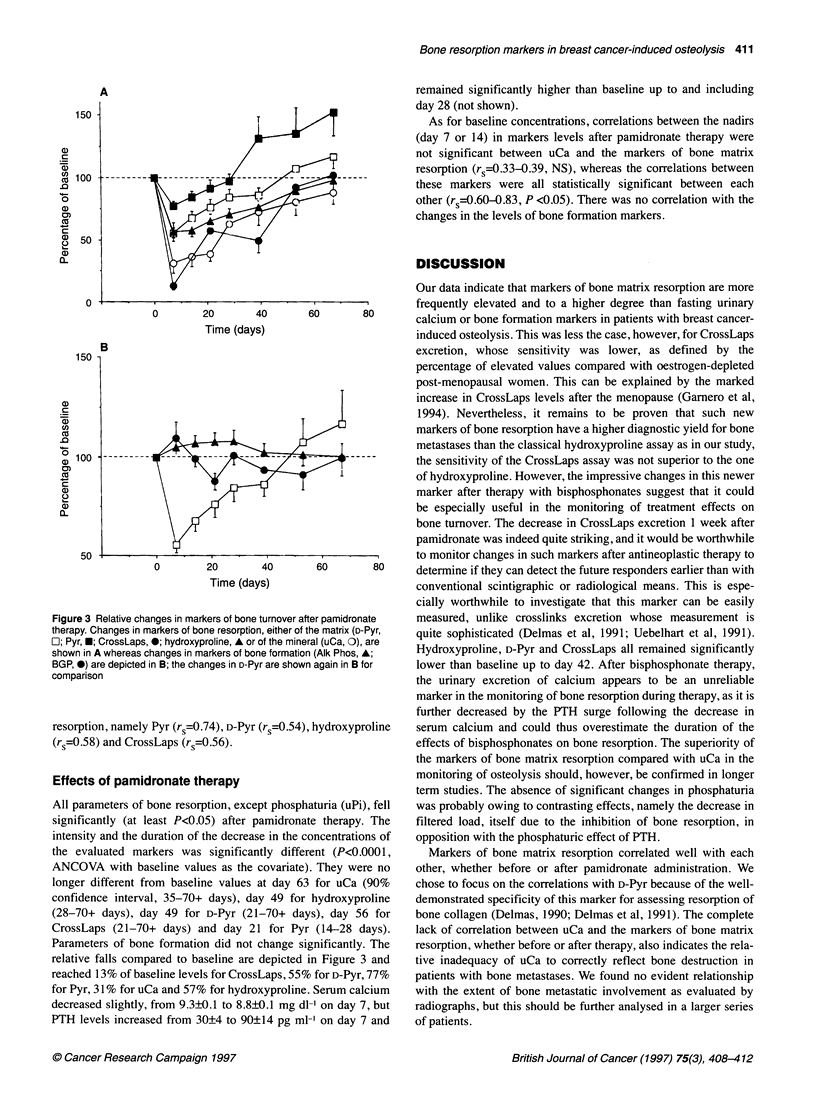
Abstract
The understanding of the pathophysiology and the monitoring of metastatic bone disease remains unsatisfactory. We compared several new markers of bone turnover in normocalcaemic patients with breast cancer-induced osteolysis before and after a single infusion of the bisphosphonate pamidronate. We studied 19 ambulatory patients with advanced breast cancer and extensive bone metastases who did not receive any systemic antineoplastic therapy. Pamidronate was administered at doses of 30, 60, 90 or 120 mg and the patients were followed weekly during a mean of 8 (range 4-10) weeks. Compared with healthy premenopausal women, the percentage of elevated values at baseline was 47% for fasting urinary calcium (uCa), 74% for hydroxyproline, 83% for CrossLaps (a new marker of type I collagen degradation) and 100% for the collagen cross-links (measured by high performance liquid chromatography), namely pyridinoline (Pyr) and deoxyPyr (D-Pyr). Pretreatment levels of uCa did not correlate significantly with any of the four markers of bone matrix resorption, whereas the correlations between these four markers were generally significant (r(s)=0.43-0.71). Alkaline phosphatase correlated significantly with markers of bone matrix resorption (r(s)=0.54-0.74). All parameters, except phosphaturia (uPi) and the bone formation markers (osteocalcin and alkaline phosphatase), fell significantly after pamidronate therapy, up to day 42 for hydroxyproline, D-Pyr and CrossLaps and day 56 for uCa. This longer lasting effect was probably due to the parathyroid hormone (PTH) surge following the decrease in serum calcium, implying that the decrease in uCa can overestimate the effects of bisphophonates on bone resorption. The decrease in bone turnover parameters was most marked for CrossLaps, indicating the potential of this new marker for monitoring therapy. Sequential determinations of markers of bone matrix resorption should be useful in delineating the optimal therapeutic schemes of bisphosphonates and for evaluating treatment effects on bone in cancer patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averbuch S. D. New bisphosphonates in the treatment of bone metastases. Cancer. 1993 Dec 1;72(11 Suppl):3443–3452. doi: 10.1002/1097-0142(19931201)72:11+<3443::aid-cncr2820721611>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Body J. J., Delmas P. D. Urinary pyridinium cross-links as markers of bone resorption in tumor-associated hypercalcemia. J Clin Endocrinol Metab. 1992 Mar;74(3):471–475. doi: 10.1210/jcem.74.3.1740478. [DOI] [PubMed] [Google Scholar]
- Body J. J., Dumon J. C., Piccart M., Ford J. Intravenous pamidronate in patients with tumor-induced osteolysis: a biochemical dose-response study. J Bone Miner Res. 1995 Aug;10(8):1191–1196. doi: 10.1002/jbmr.5650100808. [DOI] [PubMed] [Google Scholar]
- Body J. J., Dumon J. C., Seraj F., Cleeren A. Recovery of parathyroid hormone secretion during correction of tumor-associated hypercalcemia. J Clin Endocrinol Metab. 1992 Jun;74(6):1385–1388. doi: 10.1210/jcem.74.6.1592885. [DOI] [PubMed] [Google Scholar]
- Body J. J. Medical treatment of tumor-induced hypercalcemia and tumor-induced osteolysis: challenges for future research. Support Care Cancer. 1993 Jan;1(1):26–33. doi: 10.1007/BF00326636. [DOI] [PubMed] [Google Scholar]
- Body J. J. Metastatic bone disease: clinical and therapeutic aspects. Bone. 1992;13 (Suppl 1):S57–S62. doi: 10.1016/s8756-3282(09)80011-2. [DOI] [PubMed] [Google Scholar]
- Body J. J., Pot M., Borkowski A., Sculier J. P., Klastersky J. Dose/response study of aminohydroxypropylidene bisphosphonate in tumor-associated hypercalcemia. Am J Med. 1987 May;82(5):957–963. doi: 10.1016/0002-9343(87)90158-6. [DOI] [PubMed] [Google Scholar]
- Bonde M., Qvist P., Fledelius C., Riis B. J., Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem. 1994 Nov;40(11 Pt 1):2022–2025. [PubMed] [Google Scholar]
- Coleman R. E., Houston S., James I., Rodger A., Rubens R. D., Leonard R. C., Ford J. Preliminary results of the use of urinary excretion of pyridinium crosslinks for monitoring metastatic bone disease. Br J Cancer. 1992 May;65(5):766–768. doi: 10.1038/bjc.1992.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. E., Mashiter G., Whitaker K. B., Moss D. W., Rubens R. D., Fogelman I. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988 Aug;29(8):1354–1359. [PubMed] [Google Scholar]
- Delmas P. D. Biochemical markers of bone turnover for the clinical assessment of metabolic bone disease. Endocrinol Metab Clin North Am. 1990 Mar;19(1):1–18. [PubMed] [Google Scholar]
- Delmas P. D., Schlemmer A., Gineyts E., Riis B., Christiansen C. Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. J Bone Miner Res. 1991 Jun;6(6):639–644. doi: 10.1002/jbmr.5650060615. [DOI] [PubMed] [Google Scholar]
- Garnero P., Gineyts E., Riou J. P., Delmas P. D. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994 Sep;79(3):780–785. doi: 10.1210/jcem.79.3.8077361. [DOI] [PubMed] [Google Scholar]
- Lipton A., Demers L., Daniloff Y., Curley E., Hamilton C., Harvey H., Witters L., Seaman J., Van der Giessen R., Seyedin S. Increased urinary excretion of pyridinium cross-links in cancer patients. Clin Chem. 1993 Apr;39(4):614–618. [PubMed] [Google Scholar]
- Niell H. B., Palmieri G. M., Neely C. L., Maxwell T. A., Hopkins S. C., Soloway M. S. Total, dialyzable, and nondialyzable postabsorptive hydroxyproline. Values in patients with cancer. Arch Intern Med. 1983 Oct;143(10):1925–1927. [PubMed] [Google Scholar]
- Ralston S. H. Medical management of hypercalcaemia. Br J Clin Pharmacol. 1992 Jul;34(1):11–20. doi: 10.1111/j.1365-2125.1992.tb04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelhart D., Gineyts E., Chapuy M. C., Delmas P. D. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Miner. 1990 Jan;8(1):87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- Uebelhart D., Schlemmer A., Johansen J. S., Gineyts E., Christiansen C., Delmas P. D. Effect of menopause and hormone replacement therapy on the urinary excretion of pyridinium cross-links. J Clin Endocrinol Metab. 1991 Feb;72(2):367–373. doi: 10.1210/jcem-72-2-367. [DOI] [PubMed] [Google Scholar]


