Abstract
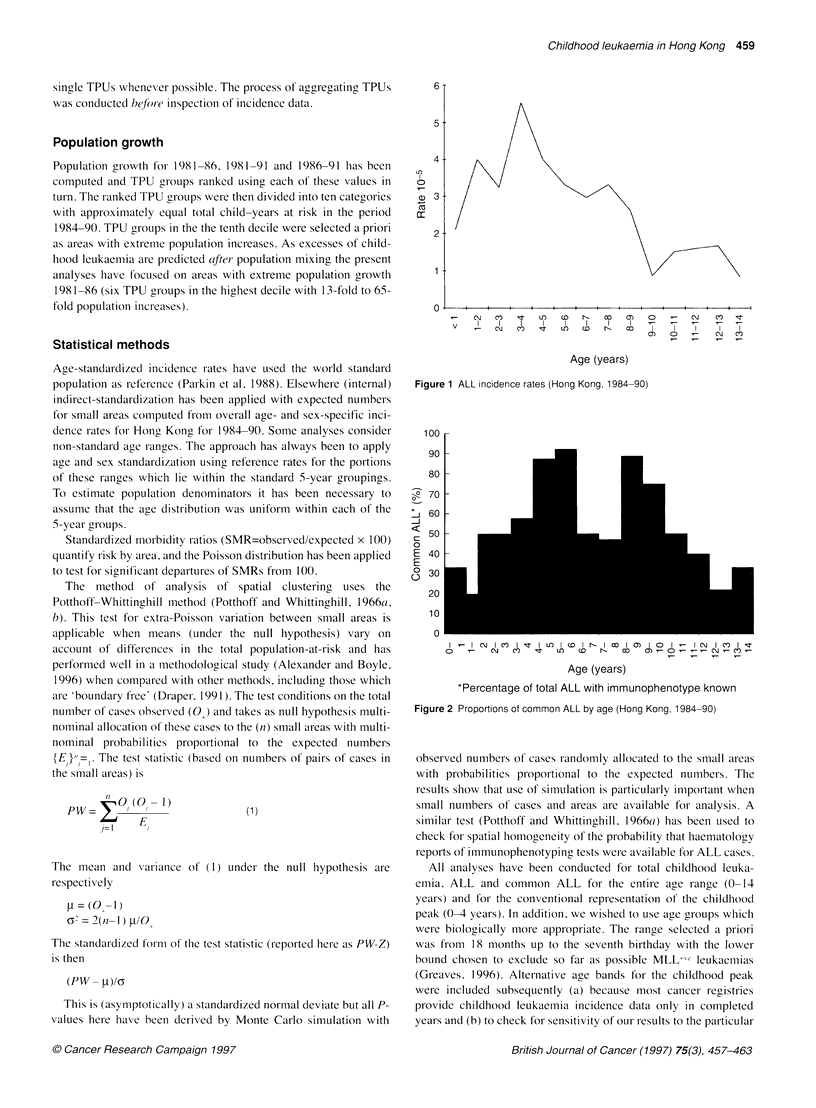
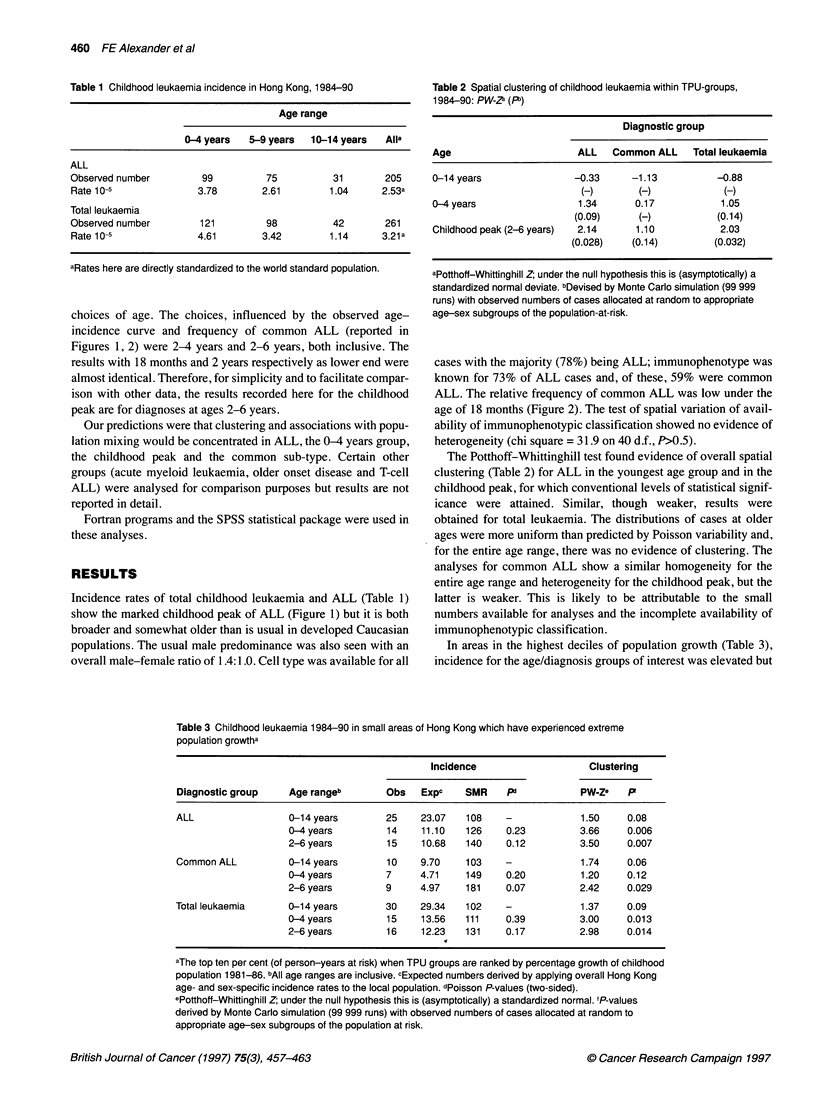
Incidence data of childhood leukaemia (CL) in Hong Kong (1984-90) have been analysed for evidence of variation between small areas. All cases (n=261) were classified by morphological cell type, with the majority (n=205) being acute lymphoblastic leukaemia (ALL), and haematological review has permitted immunophenotypic classification for 73% of these. The data have been examined for evidence of spatial clustering within small census areas (TPUs) and for association with population mixing, with attention focused on those subgroups (especially the childhood peak of ALL--taken here to be diagnoses in children from 24 months up to the seventh birthday--and common ALL) which, it has been hypothesized, may be caused by unusual patterns of exposure and response to common infections. For the whole of Hong Kong, there was evidence of spatial clustering of ALL at ages 0-4 years (P = 0.09) and in the childhood peak (P<0.05). When these analyses were restricted to TPUs where extreme population mixing may have occurred, overall incidence was elevated and significant evidence of clustering was found for ALL (P<0.007) at these ages and for the common ALL in the childhood peak (P = 0.032). Replication of the analyses for subsets of leukaemia that were not dominated by the childhood peak of ALL found no evidence of clustering. This is the first investigation of an association between population mixing and childhood leukaemia in Asia and the first to include clustering and to consider particular subsets. The results are supportive of the 'infectious' aetiology hypothesis for subsets of childhood leukaemia, specifically common ALL in the childhood peak.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan L. C., Pegram S. M., Greaves M. F. Contribution of immunophenotype to the classification and differential diagnosis of acute leukaemia. Lancet. 1985 Mar 2;1(8427):475–479. doi: 10.1016/s0140-6736(85)92085-9. [DOI] [PubMed] [Google Scholar]
- Davies D. P. Paediatric illness in Hong Kong and Britain. Arch Dis Child. 1992 Apr;67(4):543–549. doi: 10.1136/adc.67.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A. M., Ridge S. A., Cabrera M. E., Mahmoud H., Steel C. M., Chan L. C., Greaves M. In utero rearrangements in the trithorax-related oncogene in infant leukaemias. Nature. 1993 May 27;363(6427):358–360. doi: 10.1038/363358a0. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Snee M. P., Hall A. J., Powell C. A., Downes S., Terrell J. D. Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ. 1990 Feb 17;300(6722):423–429. doi: 10.1136/bmj.300.6722.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Alexander F. E. An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia. 1993 Mar;7(3):349–360. [PubMed] [Google Scholar]
- Greaves M. F., Colman S. M., Beard M. E., Bradstock K., Cabrera M. E., Chen P. M., Jacobs P., Lam-Po-Tang P. R., MacDougall L. G., Williams C. K. Geographical distribution of acute lymphoblastic leukaemia subtypes: second report of the collaborative group study. Leukemia. 1993 Jan;7(1):27–34. [PubMed] [Google Scholar]
- Greaves M. F. Infant leukaemia biology, aetiology and treatment. Leukemia. 1996 Feb;10(2):372–377. [PubMed] [Google Scholar]
- Greaves M. F., Pegram S. M., Chan L. C. Collaborative group study of the epidemiology of acute lymphoblastic leukaemia subtypes: background and first report. Leuk Res. 1985;9(6):715–733. doi: 10.1016/0145-2126(85)90281-4. [DOI] [PubMed] [Google Scholar]
- Kangro H. O., Osman H. K., Lau Y. L., Heath R. B., Yeung C. Y., Ng M. H. Seroprevalence of antibodies to human herpesviruses in England and Hong Kong. J Med Virol. 1994 May;43(1):91–96. doi: 10.1002/jmv.1890430117. [DOI] [PubMed] [Google Scholar]
- Kinlen L. J. Childhood leukaemia and non-Hodgkins lymphoma in young people living close to nuclear reprocessing sites. Biomed Pharmacother. 1993;47(10):429–434. doi: 10.1016/0753-3322(93)90338-l. [DOI] [PubMed] [Google Scholar]
- Kinlen L. J., Hudson C. M., Stiller C. A. Contacts between adults as evidence for an infective origin of childhood leukaemia: an explanation for the excess near nuclear establishments in west Berkshire? Br J Cancer. 1991 Sep;64(3):549–554. doi: 10.1038/bjc.1991.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlen L. J., O'Brien F., Clarke K., Balkwill A., Matthews F. Rural population mixing and childhood leukaemia: effects of the North Sea oil industry in Scotland, including the area near Dounreay nuclear site. BMJ. 1993 Mar 20;306(6880):743–748. doi: 10.1136/bmj.306.6880.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox E. G. Leukaemia clusters in childhood: geographical analysis in Britain. J Epidemiol Community Health. 1994 Aug;48(4):369–376. doi: 10.1136/jech.48.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linet M. S., Devesa S. S. Descriptive epidemiology of childhood leukaemia. Br J Cancer. 1991 Mar;63(3):424–429. doi: 10.1038/bjc.1991.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B. Leukemia clusters around nuclear facilities in Britain. Cancer Causes Control. 1992 May;3(3):283–288. doi: 10.1007/BF00124262. [DOI] [PubMed] [Google Scholar]
- Moore P. S., Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995 May 4;332(18):1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- Mulder Y. M., Drijver M., Kreis I. A. Case-control study on the association between a cluster of childhood haematopoietic malignancies and local environmental factors in Aalsmeer, The Netherlands. J Epidemiol Community Health. 1994 Apr;48(2):161–165. doi: 10.1136/jech.48.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou E., Kassimos D., Kalmanti M., Kosmidis H., Haidas S., Flytzani V., Tong D., Trichopoulos D. Age of exposure to infections and risk of childhood leukaemia. BMJ. 1993 Sep 25;307(6907):774–774. doi: 10.1136/bmj.307.6907.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff R. F., Whittinghill M. Testing for homogeneity. I. The binomial and multinomial distributions. Biometrika. 1966 Jun;53(1):167–182. [PubMed] [Google Scholar]
- Potthoff R. F., Whittinghill M. Testing for homogeneity. II. The Poisson distribution. Biometrika. 1966 Jun;53(1):183–190. [PubMed] [Google Scholar]
- Ramot B., Magrath I. Hypothesis: the environment is a major determinant of the immunological sub-type of lymphoma and acute lymphoblastic leukaemia in children. Br J Haematol. 1982 Feb;50(2):183–189. doi: 10.1111/j.1365-2141.1982.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Ross J. A., Potter J. D., Robison L. L. Infant leukemia, topoisomerase II inhibitors, and the MLL gene. J Natl Cancer Inst. 1994 Nov 16;86(22):1678–1680. doi: 10.1093/jnci/86.22.1678. [DOI] [PubMed] [Google Scholar]
- Taylor G. M., Robinson M. D., Binchy A., Birch J. M., Stevens R. F., Jones P. M., Carr T., Dearden S., Gokhale D. A. Preliminary evidence of an association between HLA-DPB1*0201 and childhood common acute lymphoblastic leukaemia supports an infectious aetiology. Leukemia. 1995 Mar;9(3):440–443. [PubMed] [Google Scholar]


