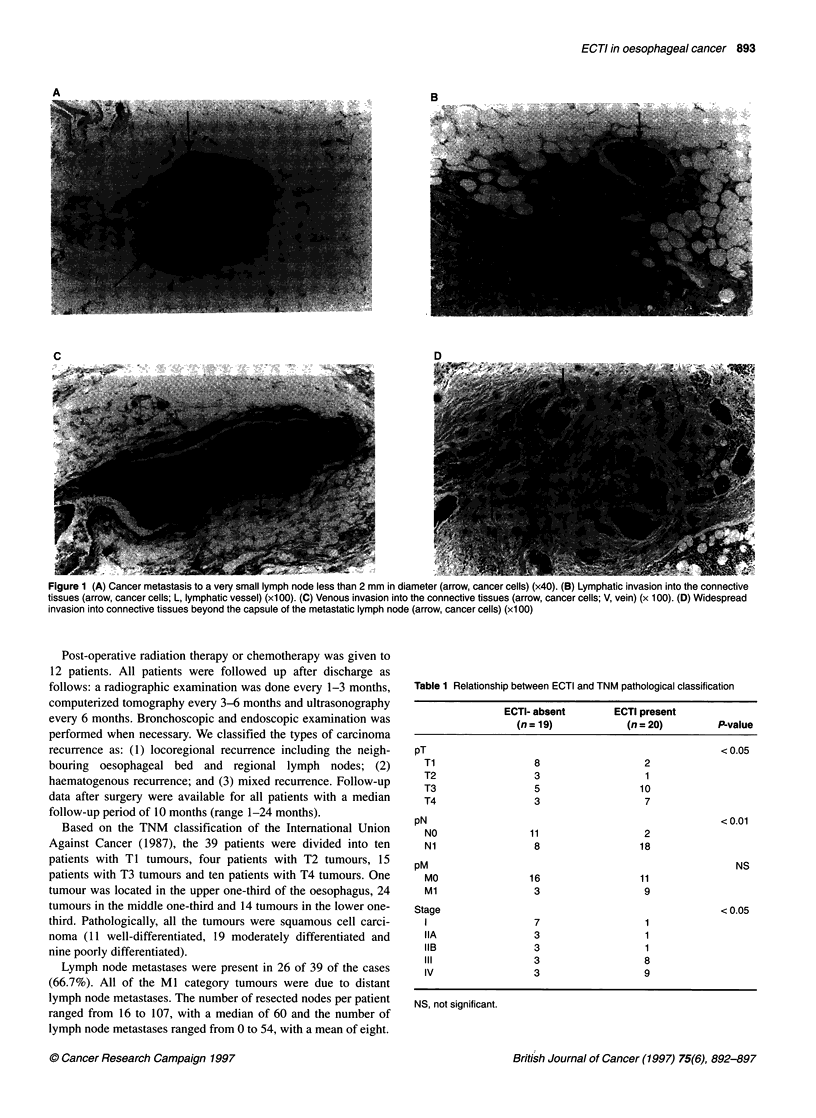
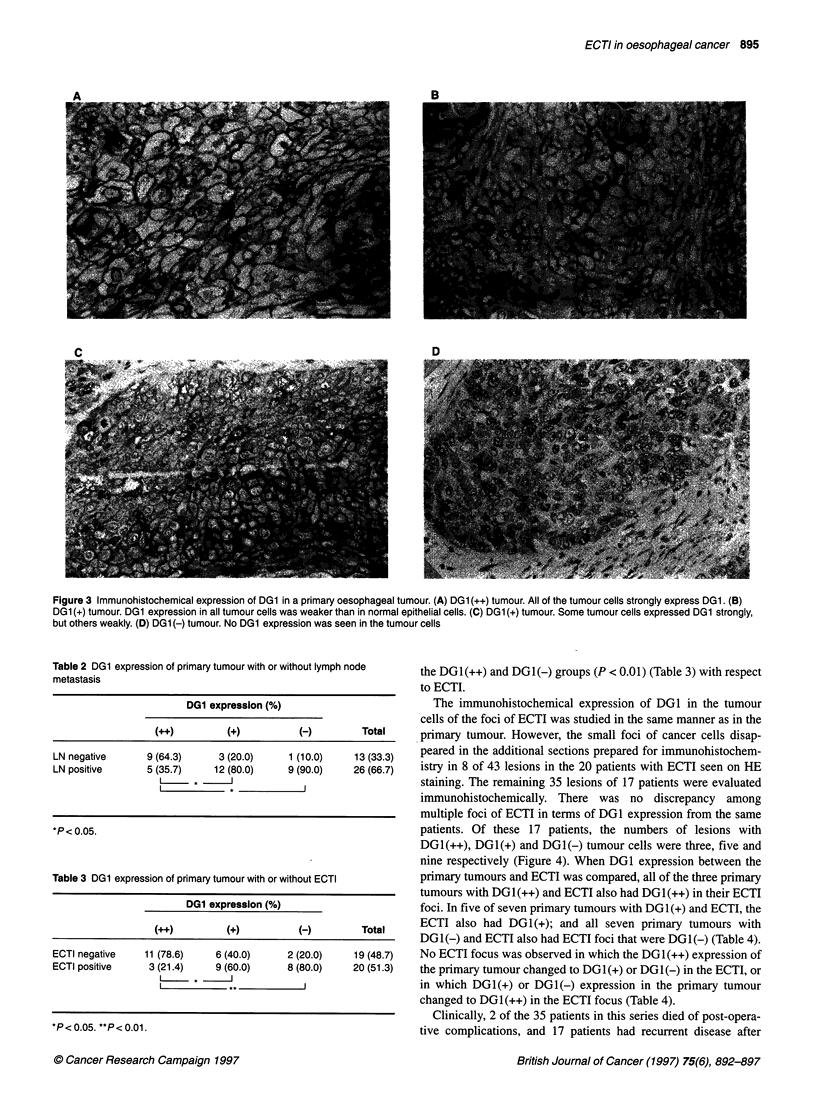
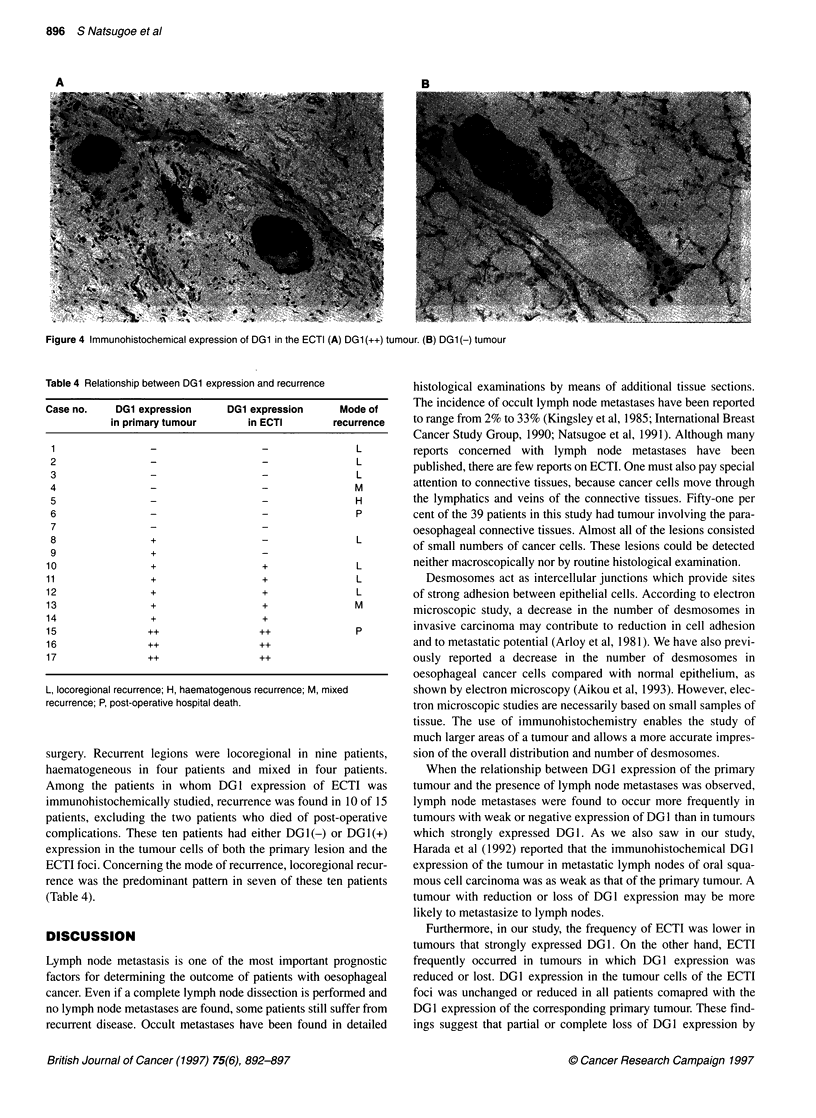
Abstract
We investigated extranodal connective tissue involvement (ECTI) in 39 patients with oesophageal carcinoma. Both the primary tumour and ECTI were immunohistochemically examined using the monoclonal antibody 32-2B for desmosomal glycoprotein 1 (DG1). Connective tissue carcinoma deposits were identified as cells within small lymph nodes, as lymphatic or venous vessel invasion or as widespread invasion beyond the capsule of metastatic lymph nodes. These histological findings were present in at least one area in 20 of 39 patients (51.3%). DG1 immunostaining intensity by tumour was graded as DG1 (++), DG1 (+) or DG1 (-). DG1 (+) or DG1 (-) primary tumours demonstrated lymph node metastases and ECTI more frequently than DG1(++) tumours (P<0.05). Among 17 patients in whom DG1 immunohistochemistry was performed on ECTI, there were three DG1(++), five DG1(+) and nine DG1(-) patients. The DG1 expression of ECTI was equal to or less intense than the primary tumour. These results indicate that reduction or loss of DG1 expression may promote ECTI and lymph node metastases. One should be aware of the potential for ECTI in oesophageal carcinomas. In the future, adjuvant therapy may be advisable for some oesophageal carcinomas based on the phenotype of individual cancer cells, including expression of DG1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Tsurumaru M., Udagawa H., Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994 Sep;220(3):364–373. doi: 10.1097/00000658-199409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy J., Pauli B. U., Weinstein R. S. Correlation between numbers of desmosomes and the aggressiveness of transitional cell carcinoma in human urinary bladder. Cancer. 1981 Jan 1;47(1):104–112. doi: 10.1002/1097-0142(19810101)47:1<104::aid-cncr2820470118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Baba M., Aikou T., Yoshinaka H., Natsugoe S., Fukumoto T., Shimazu H., Akazawa K. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg. 1994 Mar;219(3):310–316. doi: 10.1097/00000658-199403000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. W., Chan E. Y., Chan C. W. Carcinoma of the esophagus. An autopsy study of 231 cases. Pathology. 1986 Oct;18(4):400–405. doi: 10.3109/00313028609087559. [DOI] [PubMed] [Google Scholar]
- Conn I. G., Vilela M. J., Garrod D. R., Crocker J., Wallace D. M. Immunohistochemical staining with monoclonal antibody 32-2B to desmosomal glycoprotein 1. Its role in the histological assessment of urothelial carcinomas. Br J Urol. 1990 Feb;65(2):176–180. doi: 10.1111/j.1464-410x.1990.tb14694.x. [DOI] [PubMed] [Google Scholar]
- Goodwin L., Hill J. E., Raynor K., Raszi L., Manabe M., Cowin P. Desmoglein shows extensive homology to the cadherin family of cell adhesion molecules. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1224–1230. doi: 10.1016/s0006-291x(05)80917-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara A., Takahashi T., Ueda T., Iwamoto A., Yamashita H., Maeda T. Enhanced therapeutic efficacy on lymph node metastasis by the use of peplomycin adsorbed on small activated carbon particles. Anticancer Res. 1988 Mar-Apr;8(2):287–289. [PubMed] [Google Scholar]
- Harada T., Shinohara M., Nakamura S., Shimada M., Oka M. Immunohistochemical detection of desmosomes in oral squamous cell carcinomas: correlation with differentiation, mode of invasion, and metastatic potential. Int J Oral Maxillofac Surg. 1992 Dec;21(6):346–349. doi: 10.1016/s0901-5027(05)80759-3. [DOI] [PubMed] [Google Scholar]
- Kelsen D. P., Minsky B., Smith M., Beitler J., Niedzwiecki D., Chapman D., Bains M., Burt M., Heelan R., Hilaris B. Preoperative therapy for esophageal cancer: a randomized comparison of chemotherapy versus radiation therapy. J Clin Oncol. 1990 Aug;8(8):1352–1361. doi: 10.1200/JCO.1990.8.8.1352. [DOI] [PubMed] [Google Scholar]
- Kingsley W. B., Peters G. N., Cheek J. H. What constitutes adequate study of axillary lymph nodes in breast cancer? Ann Surg. 1985 Mar;201(3):311–314. doi: 10.1097/00000658-198503000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsugoe S., Aiko T., Shimazu H. A detailed histological study on occult metastasis of the lymph nodes. Jpn J Surg. 1991 Sep;21(5):528–532. doi: 10.1007/BF02470990. [DOI] [PubMed] [Google Scholar]
- Natsugoe S., Shimada M., Kumanohoso T., Tokuda K., Baba M., Yoshinaka H., Fukumoto T., Nakamura K., Yamada K., Nakashima T. Enhanced efficacy of bleomycin adsorbed on silica particles against lymph node metastasis in patients with esophageal cancer: a pilot study. Surgery. 1995 Jun;117(6):636–641. doi: 10.1016/s0039-6060(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Orringer M. B., Forastiere A. A., Perez-Tamayo C., Urba S., Takasugi B. J., Bromberg J. Chemotherapy and radiation therapy before transhiatal esophagectomy for esophageal carcinoma. Ann Thorac Surg. 1990 Mar;49(3):348–355. doi: 10.1016/0003-4975(90)90237-z. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Koch P. J., Franke W. W. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994 Apr;211(2):391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Sugimachi K., Inokuchi K., Kuwano H., Kai H., Okamura T., Okudaira Y. Patterns of recurrence after curative resection for carcinoma of the thoracic part of the esophagus. Surg Gynecol Obstet. 1983 Dec;157(6):537–540. [PubMed] [Google Scholar]
- Vilela M. J., Hashimoto T., Nishikawa T., North A. J., Garrod D. A simple epithelial cell line (MDCK) shows heterogeneity of desmoglein isoforms, one resembling pemphigus vulgaris antigen. J Cell Sci. 1995 Apr;108(Pt 4):1743–1750. doi: 10.1242/jcs.108.4.1743. [DOI] [PubMed] [Google Scholar]
- Vilela M. J., Parrish E. P., Wright D. H., Garrod D. R. Monoclonal antibody to desmosomal glycoprotein 1--a new epithelial marker for diagnostic pathology. J Pathol. 1987 Dec;153(4):365–375. doi: 10.1002/path.1711530410. [DOI] [PubMed] [Google Scholar]
- Wheeler G. N., Parker A. E., Thomas C. L., Ataliotis P., Poynter D., Arnemann J., Rutman A. J., Pidsley S. C., Watt F. M., Rees D. A. Desmosomal glycoprotein DGI, a component of intercellular desmosome junctions, is related to the cadherin family of cell adhesion molecules. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4796–4800. doi: 10.1073/pnas.88.11.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka H., Shimazu H., Natsugoe S., Haraguchi Y., Shimada M., Baba M., Fukumoto T. [Histopathological features of the lymph node metastases in patients with thoracic esophageal cancer]. Nihon Geka Gakkai Zasshi. 1992 Oct;93(10):1289–1296. [PubMed] [Google Scholar]