Abstract
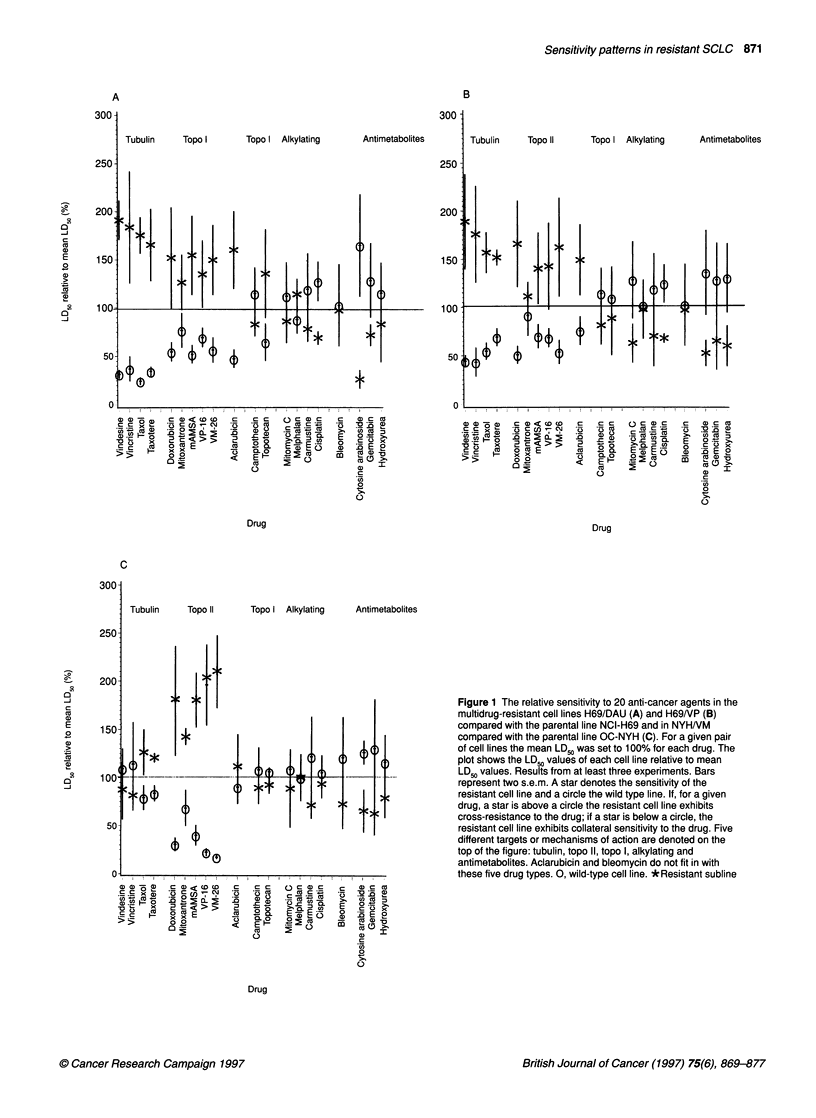
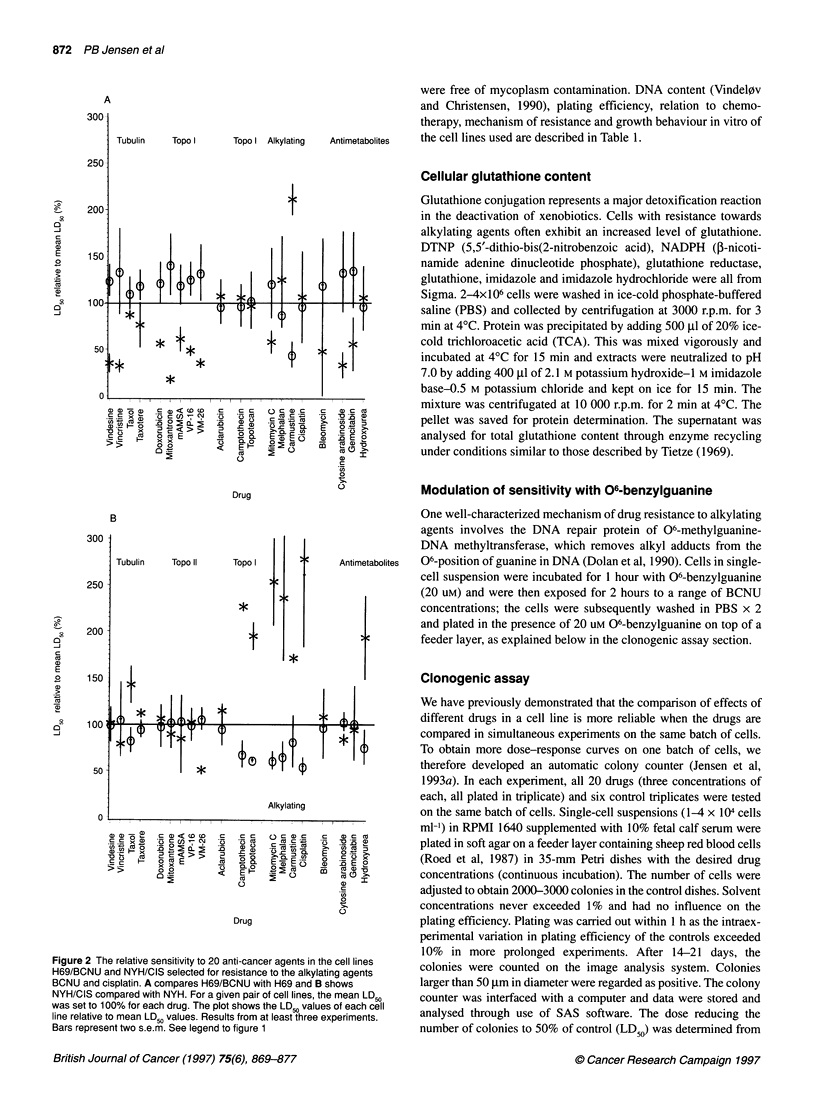
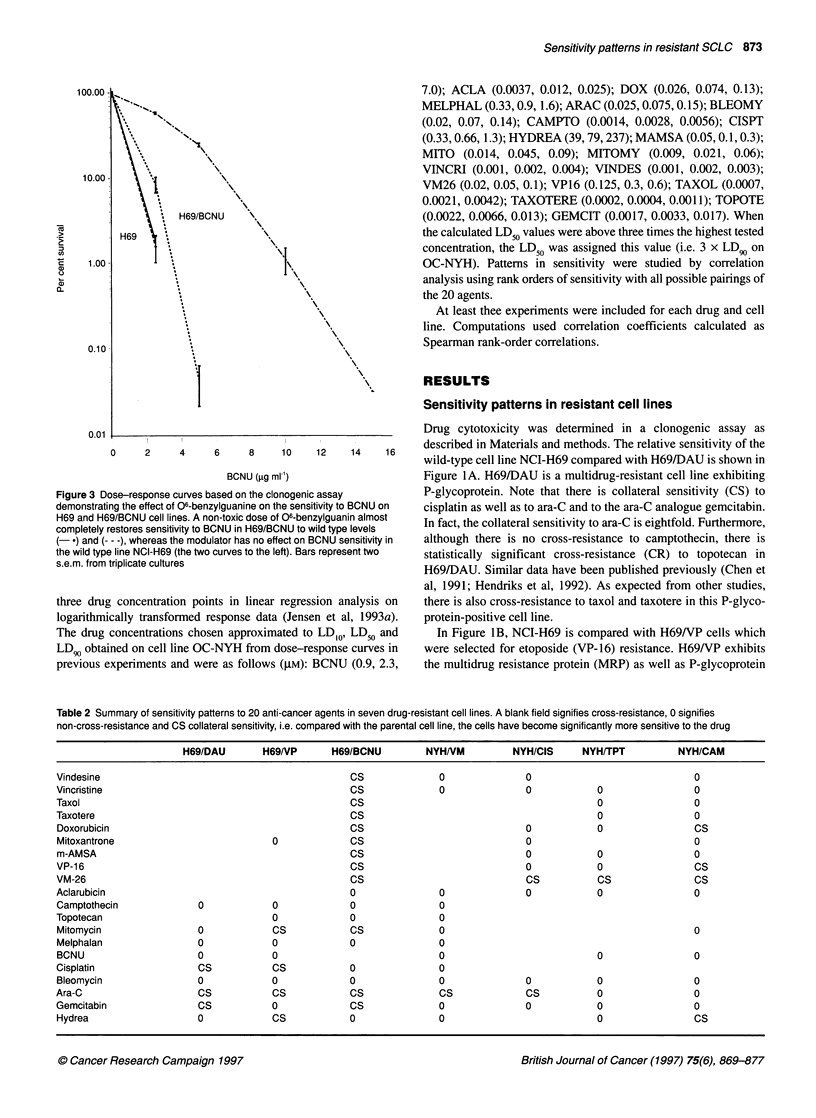
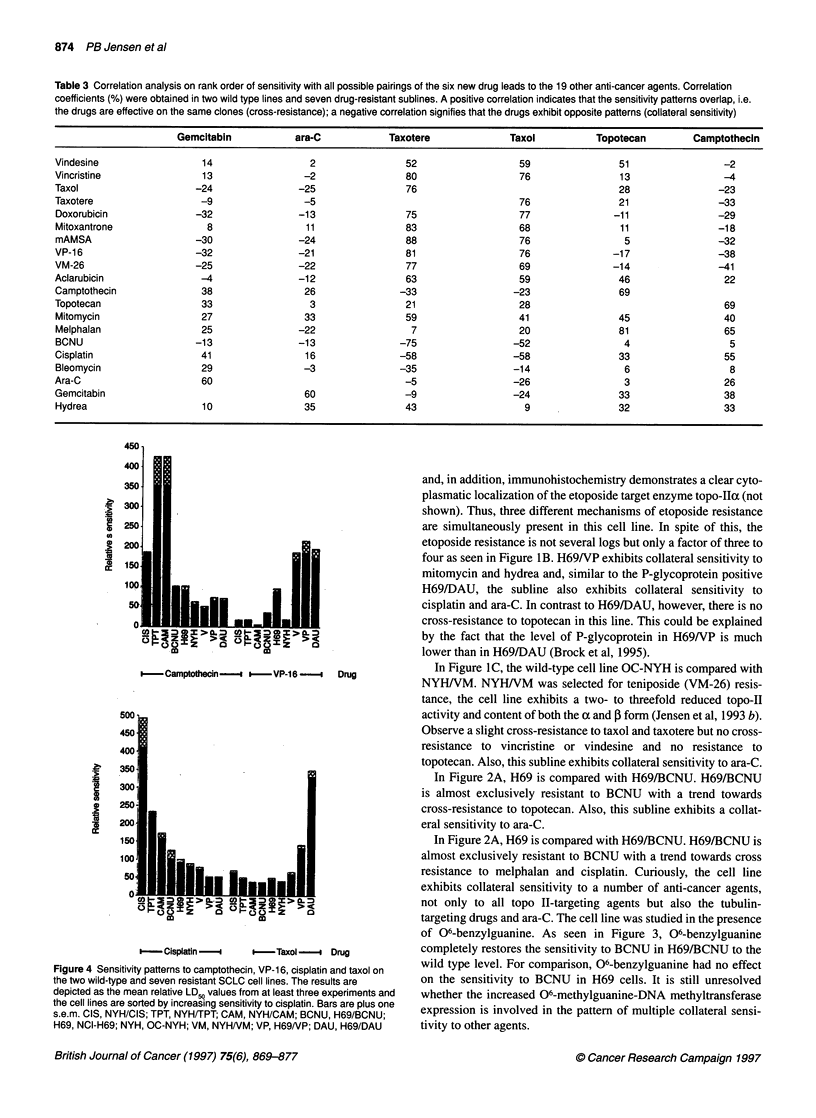
The acquisition of drug-resistant tumour cells is the main problem in the medical treatment of a range of malignant diseases. In recent years, three new classes of anti-cancer agents, each with a novel mechanism of action, have been brought forward to clinical trials. These are the topoisomerase I (topo I) poisons topotecan and irinotecan, which are both camptothecin derivatives, the taxane tubulin stabilizers taxol and taxotere and, finally, the antimetabolite gemcitabin, which is active in solid tumours. The process of optimizing their use in a combination with established agents is very complex, with numerous possible drug and schedule regimens. We describe here how a broad panel of drug-resistant small-cell lung cancer (SCLC) cell lines can be used as a model of tumour heterogeneity to aid in the selection of non-cross-resistant regimens. We have selected low-fold (3-10x) drug-resistant sublines from a classic (NCI-H69) and a variant (OC-NYH) SCLC cell line. The resistant cell lines include two sublines with different phenotypes towards alkylating agents (H69/BCNU and NYH/CIS), two sublines with different phenotypes against topo I poisons (NYH/CAM and NYH/TPT) and three multidrug resistant (MDR) sublines (H69/DAU, NYH/VM, and H69/VP) with combinations of mdr1 and MRP overexpression as well as topoisomerase II (topo II) down-regulation or mutation. Sensitivity to 20 established and new agents was measured in a standardized clonogenic assay. Resistance was highly drug specific. Thus, none of the cell lines was resistant to all drugs. In fact, all resistant cell lines exhibited patterns of collateral sensitivity to various different classes of drugs. The most intriguing pattern was collateral sensitivity to gemcitabin in two cell lines and to ara-C in five drug-resistant cell lines, i.e. in all lines except the lines resistant to topo I poisons. Next, all sensitivity patterns in the nine cell lines were compared by correlation analysis. A high correlation coefficient (CC) for a given pair of compounds indicates a similar pattern in response in the set of cell lines. Such data corroborate the view that there is cross-resistance among the drugs. A numerically low coefficient indicates that the two drugs are acting in different ways, suggesting a lack of cross-resistance between the drugs, and a negative correlation coefficient implies that two drugs exhibit collateral sensitivity. The most negative CCs (%) to the new drug leads were: taxotere-carmustine (BCNU) (-75), taxol-cisplatin (-58), ara-C-taxol (-25), gemcitabin-doxorubicin (-32), camptotecin-VM26 (-41) and topotecan-VP16 (-17). The most negative correlations to the clinically important agent VP-16 were: cisplatin (-70); BCNU (-68); camptothecin (-38); bleomycin (-33), gemcitabin (-32); ara-C (-21); topotecan (-17); melphalan (-3); and to the other main drug in SCLC treatment cisplatin were: doxorubicin (-70); VP-16 (-70); VM-26 (-69); mAMSA (-64); taxotere (-58); taxol (-58). Taxol and taxotere were highly correlated (cross-resistant) to VP-16 (0.76 and 0.81 respectively) and inversely correlated to cisplatin (both -0.58). Similarly, camptothecin and topotecan were correlated to cisplatin but inversely correlated to VP-16 and other topo II poisons. From the sensitivity data, we conclude that collateral sensitivity and lack of cross-resistance favours a cisplatin-taxane or topo I-topo II poison combination, whereas patterns of cross-resistance suggest that epipodophyllotoxin-taxane or topo I poison-cisplatin combinations may be disadvantageous.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H., Lund B., Bach F., Thatcher N., Walling J., Hansen H. H. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1994 Sep;12(9):1821–1826. doi: 10.1200/JCO.1994.12.9.1821. [DOI] [PubMed] [Google Scholar]
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belani C. P., Aisner J., Hiponia D., Engstrom C. Paclitaxel and carboplatin with and without filgrastim support in patients with metastatic non-small cell lung cancer. Semin Oncol. 1995 Aug;22(4 Suppl 9):7–12. [PubMed] [Google Scholar]
- Brock I., Hipfner D. R., Nielsen B. S., Jensen P. B., Deeley R. G., Cole S. P., Sehested M. Sequential coexpression of the multidrug resistance genes MRP and mdr1 and their products in VP-16 (etoposide)-selected H69 small cell lung cancer cells. Cancer Res. 1995 Feb 1;55(3):459–462. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Chen A. Y., Yu C., Potmesil M., Wall M. E., Wani M. C., Liu L. F. Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res. 1991 Nov 15;51(22):6039–6044. [PubMed] [Google Scholar]
- Cormier Y., Eisenhauer E., Muldal A., Gregg R., Ayoub J., Goss G., Stewart D., Tarasoff P., Wong D. Gemcitabine is an active new agent in previously untreated extensive small cell lung cancer (SCLC). A study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 1994 Mar;5(3):283–285. doi: 10.1093/oxfordjournals.annonc.a058808. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Moschel R. C., Pegg A. E. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmon K. A. Biweekly paclitaxel in the treatment of patients with metastatic breast cancer. Semin Oncol. 1995 Oct;22(5 Suppl 12):117–122. [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Hansen H. H. Management of small-cell cancer of the lung. Lancet. 1992 Apr 4;339(8797):846–849. doi: 10.1016/0140-6736(92)90287-d. [DOI] [PubMed] [Google Scholar]
- Hendricks C. B., Rowinsky E. K., Grochow L. B., Donehower R. C., Kaufmann S. H. Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue. Cancer Res. 1992 Apr 15;52(8):2268–2278. [PubMed] [Google Scholar]
- Jensen P. B., Christensen I. J., Sehested M., Hansen H. H., Vindeløv L. Differential cytotoxicity of 19 anticancer agents in wild type and etoposide resistant small cell lung cancer cell lines. Br J Cancer. 1993 Feb;67(2):311–320. doi: 10.1038/bjc.1993.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. B., Roed H., Sehested M., Demant E. J., Vindeløv L., Christensen I. J., Hansen H. H. Doxorubicin sensitivity pattern in a panel of small-cell lung-cancer cell lines: correlation to etoposide and vincristine sensitivity and inverse correlation to carmustine sensitivity. Cancer Chemother Pharmacol. 1992;31(1):46–52. doi: 10.1007/BF00695993. [DOI] [PubMed] [Google Scholar]
- Jensen P. B., Sørensen B. S., Sehested M., Demant E. J., Kjeldsen E., Friche E., Hansen H. H. Different modes of anthracycline interaction with topoisomerase II. Separate structures critical for DNA-cleavage, and for overcoming topoisomerase II-related drug resistance. Biochem Pharmacol. 1993 May 25;45(10):2025–2035. doi: 10.1016/0006-2952(93)90013-m. [DOI] [PubMed] [Google Scholar]
- Jensen P. B., Vindeløv L., Roed H., Demant E. J., Sehested M., Skovsgaard T., Hansen H. H. In vitro evaluation of the potential of aclarubicin in the treatment of small cell carcinoma of the lung (SCCL). Br J Cancer. 1989 Dec;60(6):838–844. doi: 10.1038/bjc.1989.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen E., Bonven B. J., Andoh T., Ishii K., Okada K., Bolund L., Westergaard O. Characterization of a camptothecin-resistant human DNA topoisomerase I. J Biol Chem. 1988 Mar 15;263(8):3912–3916. [PubMed] [Google Scholar]
- Koutsoukos A. D., Rubinstein L. V., Faraggi D., Simon R. M., Kalyandrug S., Weinstein J. N., Kohn K. W., Paull K. D. Discrimination techniques applied to the NCI in vitro anti-tumour drug screen: predicting biochemical mechanism of action. 1994 Mar 15-Apr 15Stat Med. 13(5-7):719–730. doi: 10.1002/sim.4780130532. [DOI] [PubMed] [Google Scholar]
- Lefevre D., Riou J. F., Ahomadegbe J. C., Zhou D. Y., Benard J., Riou G. Study of molecular markers of resistance to m-AMSA in a human breast cancer cell line. Decrease of topoisomerase II and increase of both topoisomerase I and acidic glutathione S transferase. Biochem Pharmacol. 1991 Jun 15;41(12):1967–1979. doi: 10.1016/0006-2952(91)90138-u. [DOI] [PubMed] [Google Scholar]
- McGuire W. P., Hoskins W. J., Brady M. F., Kucera P. R., Partridge E. E., Look K. Y., Clarke-Pearson D. L., Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996 Jan 4;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- Oguro M., Seki Y., Okada K., Andoh T. Collateral drug sensitivity induced in CPT-11 (a novel derivative of camptothecin)-resistant cell lines. Biomed Pharmacother. 1990;44(4):209–216. doi: 10.1016/0753-3322(90)90026-6. [DOI] [PubMed] [Google Scholar]
- Roed H., Christensen I. B., Vindeløv L. L., Spang-Thomsen M., Hansen H. H. Inter-experiment variation and dependence on culture conditions in assaying the chemosensitivity of human small cell lung cancer cell lines. Eur J Cancer Clin Oncol. 1987 Feb;23(2):177–186. doi: 10.1016/0277-5379(87)90012-5. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr, Skipper H. E., Trader M. W., Laster W. R., Jr, Griswold D. P., Jr, Corbett T. H. Establishment of cross-resistance profiles for new agents. Cancer Treat Rep. 1983 Oct;67(10):905–922. [PubMed] [Google Scholar]
- Sehested M., Friche E., Jensen P. B., Demant E. J. Relationship of VP-16 to the classical multidrug resistance phenotype. Cancer Res. 1992 May 15;52(10):2874–2879. [PubMed] [Google Scholar]
- Sorensen M., Sehested M., Jensen P. B. Characterisation of a human small-cell lung cancer cell line resistant to the DNA topoisomerase I-directed drug topotecan. Br J Cancer. 1995 Aug;72(2):399–404. doi: 10.1038/bjc.1995.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Tsukahara S., Oh-hara T., Liu L. F., Tsuruo T. Elevated expression of DNA topoisomerase II in camptothecin-resistant human tumor cell lines. Cancer Res. 1990 Dec 15;50(24):7962–7965. [PubMed] [Google Scholar]
- Tan K. B., Mattern M. R., Boyce R. A., Schein P. S. Elevated DNA topoisomerase II activity in nitrogen mustard-resistant human cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7668–7671. doi: 10.1073/pnas.84.21.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa A., Pommier Y. Topoisomerase I alteration in a camptothecin-resistant cell line derived from Chinese hamster DC3F cells in culture. Cancer Res. 1992 Apr 1;52(7):1848–1854. [PubMed] [Google Scholar]
- Tsai C. M., Ihde D. C., Kadoyama C., Venzon D., Gazdar A. F. Correlation of in vitro drug sensitivity testing of long-term small cell lung cancer cell lines with response and survival. Eur J Cancer. 1990;26(11-12):1148–1152. doi: 10.1016/0277-5379(90)90274-w. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Kohn K. W., Grever M. R., Viswanadhan V. N., Rubinstein L. V., Monks A. P., Scudiero D. A., Welch L., Koutsoukos A. D., Chiausa A. J. Neural computing in cancer drug development: predicting mechanism of action. Science. 1992 Oct 16;258(5081):447–451. doi: 10.1126/science.1411538. [DOI] [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]


