Abstract
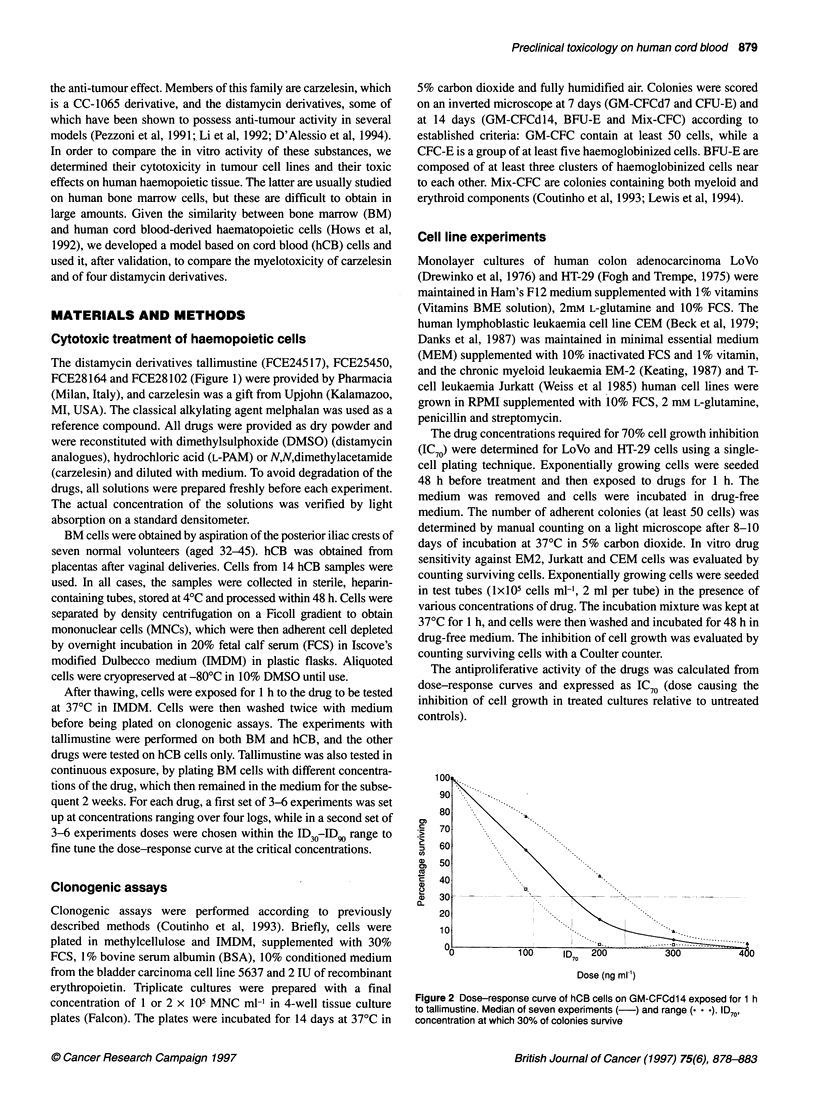
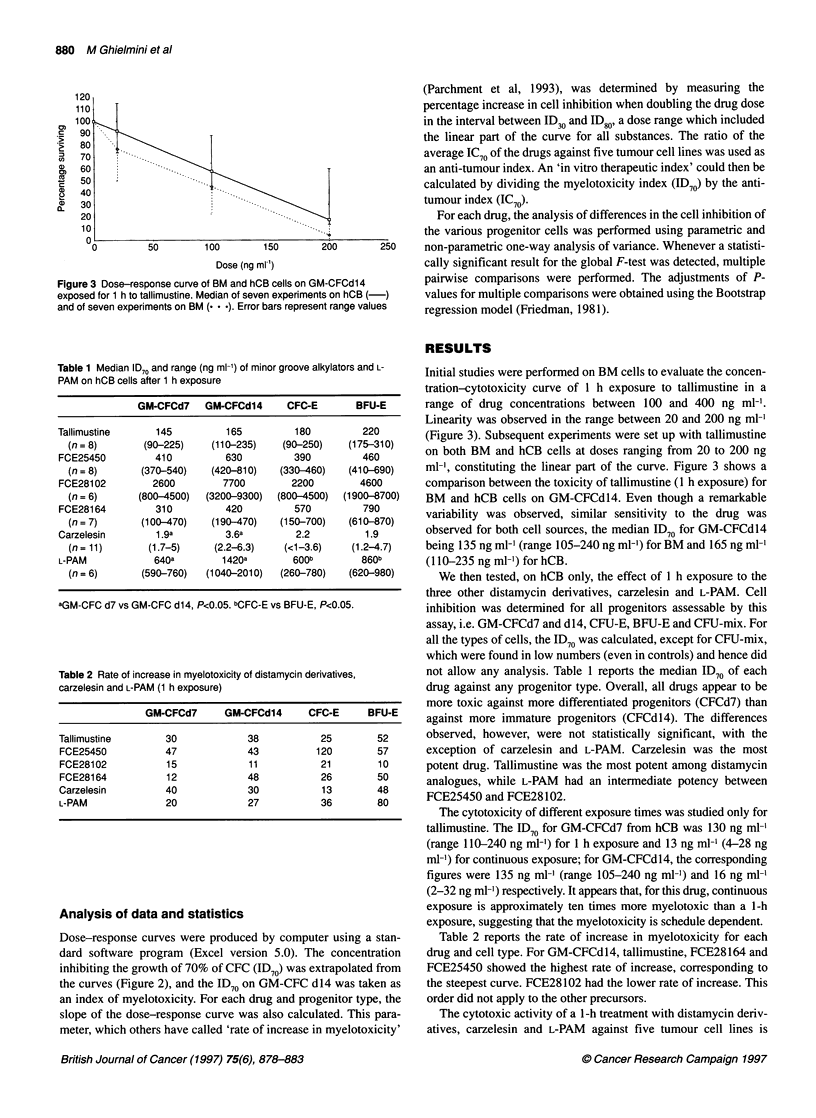
We evaluated the myelotoxicity and the anti-tumor potential of tallimustine, three of its analogues and carzelesin, with melphalan as reference substance. Tallimustine was tested by clonogenic assays on both human bone marrow (BM) and cord blood (hCB) cells, the other compounds on hCB only. The degree of inhibition of the haemopoietic progenitors GM-CFC, CFC-E and BFU-E was evaluated after exposure to different concentrations. The same schedules were tested on five tumour cell lines. We found that the dose-response curves for tallimustine on BM and hCB cells were similar. Carzelesin was shown to be the most potent of the substances tested and to be the one with the best in vitro therapeutic index; of the distamycin analogues, the one bearing an alpha-bromoacrylic group (FCE 25450) had the best index. For melphalan, tallimustine and carzelesin, the concentration inhibiting the growth of 70% of progenitor cells in vitro (ID70) was similar to the concentrations found in the serum of patients treated at the maximum tolerated dose (MTD). We conclude that hCB cells may be used instead of BM cells for in vitro myelotoxicity tests. Therapeutic indexes can be extrapolated from this model and could help in selecting the most promising analogue for further clinical development. The in vitro-active concentrations are similar to myelotoxic concentrations in patients, suggesting a predictive value for the assay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broggini M., Erba E., Ponti M., Ballinari D., Geroni C., Spreafico F., D'Incalci M. Selective DNA interaction of the novel distamycin derivative FCE 24517. Cancer Res. 1991 Jan 1;51(1):199–204. [PubMed] [Google Scholar]
- Collins J. M., Grieshaber C. K., Chabner B. A. Pharmacologically guided phase I clinical trials based upon preclinical drug development. J Natl Cancer Inst. 1990 Aug 15;82(16):1321–1326. doi: 10.1093/jnci/82.16.1321. [DOI] [PubMed] [Google Scholar]
- D'Incalci M. DNA-minor-groove alkylators, a new class of anticancer agents. Ann Oncol. 1994 Dec;5(10):877–878. doi: 10.1093/oxfordjournals.annonc.a058724. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Yalowich J. C., Beck W. T. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987 Mar 1;47(5):1297–1301. [PubMed] [Google Scholar]
- Drewinko B., Romsdahl M. M., Yang L. Y., Ahearn M. J., Trujillo J. M. Establishment of a human carcinoembryonic antigen-producing colon adenocarcinoma cell line. Cancer Res. 1976 Feb;36(2 Pt 1):467–475. [PubMed] [Google Scholar]
- Du D. L., Volpe D. A., Grieshaber C. K., Murphy M. J., Jr Comparative toxicity of fostriecin, hepsulfam and pyrazine diazohydroxide to human and murine hematopoietic progenitor cells in vitro. Invest New Drugs. 1991 May;9(2):149–157. doi: 10.1007/BF00175082. [DOI] [PubMed] [Google Scholar]
- Du D. L., Volpe D. A., Grieshaber C. K., Murphy M. J., Jr Effects of L-phenylalanine mustard and L-buthionine sulfoximine on murine and human hematopoietic progenitor cells in vitro. Cancer Res. 1990 Jul 1;50(13):4038–4043. [PubMed] [Google Scholar]
- Du X. X., Scott D., Yang Z. X., Cooper R., Xiao X. L., Williams D. A. Interleukin-11 stimulates multilineage progenitors, but not stem cells, in murine and human long-term marrow cultures. Blood. 1995 Jul 1;86(1):128–134. [PubMed] [Google Scholar]
- Fritsch G., Stimpfl M., Kurz M., Printz D., Buchinger P., Fischmeister G., Hoecker P., Gadner H. The composition of CD34 subpopulations differs between bone marrow, blood and cord blood. Bone Marrow Transplant. 1996 Feb;17(2):169–178. [PubMed] [Google Scholar]
- Gianni L., Viganò L., Surbone A., Ballinari D., Casali P., Tarella C., Collins J. M., Bonadonna G. Pharmacology and clinical toxicity of 4'-iodo-4'-deoxydoxorubicin: an example of successful application of pharmacokinetics to dose escalation in phase I trials. J Natl Cancer Inst. 1990 Mar 21;82(6):469–477. doi: 10.1093/jnci/82.6.469. [DOI] [PubMed] [Google Scholar]
- Hao Q. L., Shah A. J., Thiemann F. T., Smogorzewska E. M., Crooks G. M. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995 Nov 15;86(10):3745–3753. [PubMed] [Google Scholar]
- Hows J. M., Bradley B. A., Marsh J. C., Luft T., Coutinho L., Testa N. G., Dexter T. M. Growth of human umbilical-cord blood in longterm haemopoietic cultures. Lancet. 1992 Jul 11;340(8811):73–76. doi: 10.1016/0140-6736(92)90396-k. [DOI] [PubMed] [Google Scholar]
- Keating A. Ph positive CML cell lines. Baillieres Clin Haematol. 1987 Dec;1(4):1021–1029. doi: 10.1016/s0950-3536(87)80037-9. [DOI] [PubMed] [Google Scholar]
- Lewis J. L., Blackett N. M., Gordon M. Y. The kinetics of colony formation by CFU-GM in vitro. Br J Haematol. 1994 Oct;88(2):440–442. doi: 10.1111/j.1365-2141.1994.tb05052.x. [DOI] [PubMed] [Google Scholar]
- Li L. H., DeKoning T. F., Kelly R. C., Krueger W. C., McGovren J. P., Padbury G. E., Petzold G. L., Wallace T. L., Ouding R. J., Prairie M. D. Cytotoxicity and antitumor activity of carzelesin, a prodrug cyclopropylpyrroloindole analogue. Cancer Res. 1992 Sep 15;52(18):4904–4913. [PubMed] [Google Scholar]
- Léglise M. C., Darodes de Tailly P., Vignot J. L., Le Bot M. A., Le Roux A. M., Riché C. A cellular model for drug interactions on hematopoiesis: the use of human umbilical cord blood progenitors as a model for the study of drug-related myelosuppression of normal hematopoiesis. Cell Biol Toxicol. 1996 Feb;12(1):39–53. doi: 10.1007/BF00143393. [DOI] [PubMed] [Google Scholar]
- Parchment R. E., Huang M., Erickson-Miller C. L. Roles for in vitro myelotoxicity tests in preclinical drug development and clinical trial planning. Toxicol Pathol. 1993;21(2):241–250. doi: 10.1177/019262339302100217. [DOI] [PubMed] [Google Scholar]
- Parchment R. E., Volpe D. A., LoRusso P. M., Erickson-Miller C. L., Murphy M. J., Jr, Grieshaber C. K. In vivo-in vitro correlation of myelotoxicity of 9-methoxypyrazoloacridine (NSC-366140, PD115934) to myeloid and erythroid hematopoietic progenitors from human, murine, and canine marrow. J Natl Cancer Inst. 1994 Feb 16;86(4):273–280. doi: 10.1093/jnci/86.4.273. [DOI] [PubMed] [Google Scholar]
- Pezzoni G., Grandi M., Biasoli G., Capolongo L., Ballinari D., Giuliani F. C., Barbieri B., Pastori A., Pesenti E., Mongelli N. Biological profile of FCE 24517, a novel benzoyl mustard analogue of distamycin A. Br J Cancer. 1991 Dec;64(6):1047–1050. doi: 10.1038/bjc.1991.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky E. K., Noe D. A., Grochow L. B., Sartorious S. E., Bowling M. K., Chen T. L., Lubejko B. G., Kaufmann S. H., Donehower R. C. Phase I and pharmacologic studies of pyrazoloacridine, a novel DNA intercalating agent, on single-dosing and multiple-dosing schedules. J Clin Oncol. 1995 Aug;13(8):1975–1984. doi: 10.1200/JCO.1995.13.8.1975. [DOI] [PubMed] [Google Scholar]
- Sessa C., Pagani O., Zurlo M. G., de Jong J., Hofmann C., Lassus M., Marrari P., Strolin Benedetti M., Cavalli F. Phase I study of the novel distamycin derivative tallimustine (FCE 24517). Ann Oncol. 1994 Dec;5(10):901–907. doi: 10.1093/oxfordjournals.annonc.a058728. [DOI] [PubMed] [Google Scholar]
- Volpe D. A., Du D. L., Zurlo M. G., Mongelli N., Murphy M. J. Comparative in vitro myelotoxicity of FCE 24517, a distamycin derivative, to human, canine and murine hematopoietic progenitor cells. Invest New Drugs. 1992 Nov;10(4):255–261. doi: 10.1007/BF00944178. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Zucchetti M., D'Incalci M., Willems Y., Cavalli F., Sessa C. Lack of effect of cisplatin on i.v. L-PAM plasma pharmacokinetics in ovarian cancer patients. Cancer Chemother Pharmacol. 1988;22(1):87–89. doi: 10.1007/BF00254189. [DOI] [PubMed] [Google Scholar]


