Abstract
Cardiac pacemaking involves a variety of ion channels, but their relative importance is controversial and remains to be determined. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels, which underlie the If current of sinoatrial cells, are thought to be key players in cardiac automaticity. In addition, the increase in heart rate following beta-adrenergic stimulation has been attributed to the cAMP-mediated enhancement of HCN channel activity. We have now studied mice in which the predominant sinoatrial HCN channel isoform HCN4 was deleted in a temporally controlled manner. Here, we show that deletion of HCN4 in adult mice eliminates most of sinoatrial If and results in a cardiac arrhythmia characterized by recurrent sinus pauses. However, the mutants show no impairment in heart rate acceleration during sympathetic stimulation. Our results reveal that unexpectedly the channel does not play a role for the increase of the heart rate; however, HCN4 is necessary for maintaining a stable cardiac rhythm, especially during the transition from stimulated to basal cardiac states.
Keywords: arrhythmia, HCN4, hyperpolarization-activated channels, pacemaking, sinoatrial node
Introduction
The normal cardiac rhythm is generated in the sinoatrial node (SAN), where an interplay between depolarizing and repolarizing currents creates stable pacemaking activity. Dysfunction of the SAN results in various cardiac arrhythmias requiring pharmacological therapy or implantation of electronic pacemakers. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels, which mediate the If current in the heart (and Ih in neurons) (Ludwig et al, 1998; Kaupp and Seifert, 2001; Biel et al, 2002; Robinson and Siegelbaum, 2003) are thought to be key players in generating and regulating pacemaker activity. These channels are modulated by direct binding of cAMP (DiFrancesco and Tortora, 1991; Wainger et al, 2001). Stimulation of beta-adrenergic receptors in sinoatrial cells accelerates the heart rate by increasing intracellular cAMP levels. It has been assumed that the cAMP-mediated enhancement of HCN channel activity is largely responsible for this increase in heart rate (Brown et al, 1979; DiFrancesco, 1993; Zagotta et al, 2003). However, the precise role of HCN channels for cardiac automaticity and heart-rate modulation is controversial (Vassalle, 1995; Vinogradova et al, 2005) and remains to be determined. Four genes encoding HCN channels have been identified (HCN1–4). Several studies have shown that HCN4 is the most highly expressed HCN isoform in the sinoatrial node (Ishii et al, 1999; Shi et al, 1999; Moosmang et al, 2001; Liu et al, 2007).
Here, we have investigated the role of HCN4 for cardiac pacemaking by combining a temporally controlled gene-knockout approach with analyses of heart rate and rhythm in intact animals and electrophysiological recordings from isolated hearts and SAN cells. Contrary to expectations, we find that the channel does not play a role in the beta-adrenergic-induced increase in heart rate. Our results rather suggest that HCN4 functions as a depolarization reserve supporting pacemaking in certain critical physiological states.
Results
Generation of adult mice lacking HCN4 channels
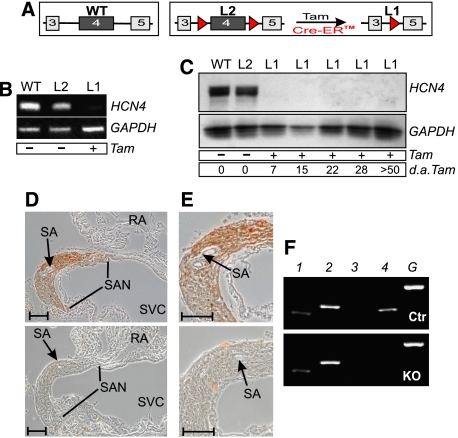
Mice lacking the HCN4 channel globally die in utero at embryonic days 10.5–11.5 (Stieber et al, 2003). To study the role of the channel in adult animals, we attempted to delete HCN4 in a conditional manner using the Cre/loxP-system. We first analyzed six Cre transgenic lines, the heart-specific inducible MerCreMer (Sohal et al, 2001) and MHC-Cre (Minamino et al, 2001), the ubiquitous inducible ROSA-Cre (Vooijs et al, 2001), GTEV-Cre (Vallier et al, 2001) and CAGG-Cre (Hayashi and McMahon, 2002) and the muscle-specific non-inducible MCK-Cre (Bruning et al, 1998), for their recombination efficiency in cardiac pacemaker cells (Supplementary Table S1 and Figure S1). These lines were crossed to a lacZ reporter strain (Akagi et al, 1997) and sections through the sinoatrial node were analyzed by X-Gal staining. Cre lines demonstrating appropriate staining were subsequently mated with floxed HCN4 mice (HCN4L2, Figure 1A) for further investigation of HCN4 deletion. Out of the six transgenes analyzed, only the CAGGCre-ER™-line (Hayashi and McMahon, 2002), which expresses a tamoxifen-inducible Cre construct, allowed complete recombination of the floxed HCN4 locus. At the age of 8 weeks, double mutants carrying CAGGCre-ER™ and HCN4L2 alleles were treated with tamoxifen (Tam). This treatment resulted in the ablation of HCN4 transcript in the SAN (Figure 1B). Western blot and immunohistochemistry using an HCN4-specific antibody further demonstrated the complete loss of HCN4 protein in the SAN (Figure 1C–E). Immunoblot analysis of sinoatrial protein, prepared at different time points after Tam injection, indicated that the protein was fully deleted as soon as 1 week after Tam treatment (Figure 1C). The deletion of HCN4 had no effect on the morphology of the SAN (Figure 1D and E).
Figure 1.
Temporally controlled deletion of HCN4 in the primary cardiac conduction system. (A) Schematic representation of wild-type (WT), floxed (L2) and HCN4-knockout (L1) alleles. Numbered boxes indicate exons 3–5. LoxP sites are represented by red triangles. Activation of the Tam-inducible Cre recombinase (Cre-ER™) results in the deletion of exon 4, encoding pore and S6 segment of the channel. (B–E) Tam-induced disruption of HCN4 in the sinoatrial node. (B) RT–PCR analysis. Total RNA was prepared from sinoatrial node tissue of wild-type and double-transgenic animals before (L2) and after (L1) Tam treatment. HCN4, amplicon corresponding to HCN4 wild-type RNA; GAPDH, internal control. (C) Immunoblot analysis. At different time points, sinoatrial proteins were isolated from wild-type and double transgenic animals before (L2) and after (L1) injection of Tam. The blot was probed with an anti-HCN4 antibody. A GAPDH antibody was used to check for equal loading. d.a.Tam., days after last injection of Tam. (D) Immunohistochemical detection of HCN4. Transverse sections through the sinoatrial node region of a control (upper panel) and a Tam-treated double-transgenic (lower panel) animal labeled with anti-HCN4 antibody. RA, right atrium; SA, sinoatrial node artery; SAN, sinoatrial node; SVC, superior vena cava. (E) Higher-magnification images of the sections shown in (D). Scale bars in (D, E), 100 μm. (F) RT–PCR using RNA prepared from the SAN area shows no change in the expression level of the remaining HCN isoforms in knockout compared to control animals. 1, HCN1; 2, HCN2; 3, HCN3; 4, HCN4; G, GAPDH.
No upregulation of other HCN channels in sinoatrial node cells of HCN4-knockout mice
As has been demonstrated for several species, HCN4 is the main HCN subtype in the SAN. In addition, sinoatrial expression of other HCN isoforms has been described (Ishii et al, 1999; Shi et al, 1999; Moosmang et al, 2001; Liu et al, 2007). Therefore, the lack of one HCN subtype could be partially or totally compensated by the upregulation of other HCN isoforms. However, the persistent reduction of If in isolated SAN cells of HCN4-knockout mice, described below, argues against this compensatory mechanism. Nevertheless, to evaluate this possibility, we first analyzed the expression of all four HCN isoforms in wild-type SAN by semiquantitative RT–PCR and found transcripts for HCN1, 2 and 4 (Figure 1F, upper panel). The low-level expression of HCN1 and HCN2 in sinoatrial myocytes was confirmed by in situ hybridization of sections through the nodal area and by immunolabeling of isolated SAN cells (Supplementary Figure S2).
The expression level of these isoforms in the primary pacemaker system was not altered by the temporally controlled deletion of HCN4 as determined by semiquantitative RT–PCR (Figure 1F, lower panel). Additionally, we performed quantitative RT–PCR analyses, using mRNA isolated from sinoatrial node tissue, to corroborate this finding. Again, no upregulation of HCN subtypes could be detected (Supplementary Figure S3).
Expression of various genes related to cardiac pacemaking is not altered in HCN4-deficient mice
As not only the expression level of other HCN subtypes could have been modified by the deletion of HCN4, we examined the expression of several other genes implicated to play important roles in cardiac pacemaking. As for mechanisms that could underlie the spontaneous depolarization in SAN cells, we chose the L-Type calcium channels Cav1.2 and Cav1.3, the T-type calcium channel Cav 3.1, the ryanodine receptor RyR2 and the Na+/Ca2+ exchanger NCX1 (Irisawa et al, 1993; Noma, 1996; Schram et al, 2002; Vinogradova et al, 2005; Mangoni et al, 2006) for further investigation. In addition, because a reduction in repolarizing currents could also contribute to an enhanced pacemaker activity, expression levels of the potassium channels Kir2.1, Kir3.1, mERG, KvLQt1 and MiRP1 (Wickman et al, 1998; Lei et al, 2001; Miake et al, 2002; Verheijck et al, 2002; Dibb et al, 2003; Yu et al, 2004) were analyzed using quantitative RT–PCR assays. We found no differences in the expression of these genes between wild-type and knockout SAN (Supplementary Figure S3).
As many depolarizing and repolarizing mechanisms are regulated by phosphorylation, we analyzed the phosphorylation level of Phospholamban as an indicator of PKA activity. The nodal protein level of phospho-Phospholamban did not differ between control and knockout animals (Supplementary Figure S3).
If in knockout sinoatrial node cells is reduced and has HCN2 and HCN1-like features
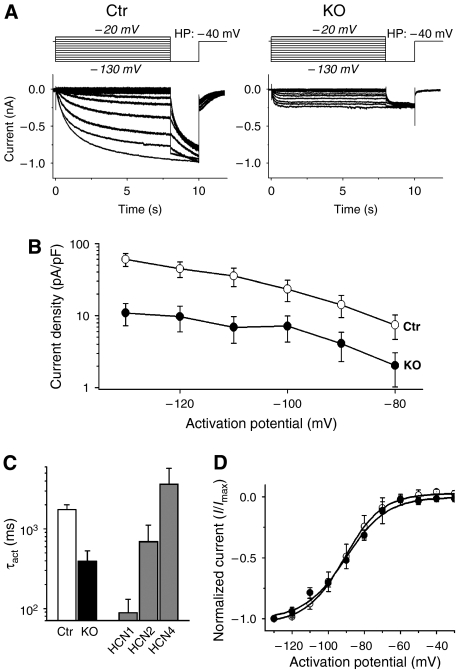
We next analyzed If in isolated sinoatrial cells. No difference in morphology or cell capacitance between control and mutant cells was observed (Supplementary Figure S4). Compared to controls, If in knockout sinoatrial cells was reduced on average by about 75% over the range of activation potentials analyzed (−80 to −130 mV) (Figure 2A and B and Supplementary Figure S5). As both the kinetics and voltage-dependence of activation differ between the various HCN isoforms (Kaupp and Seifert, 2001; Robinson and Siegelbaum, 2003; Ludwig et al, 2004; Stieber et al, 2005), we compared the activation parameters of the sinoatrial If with murine HCN1, 2 and 4 expressed in HEK293 cells under identical conditions to roughly define the subtypes underlying the small residual If in knockout cells. The time constant of activation of the residual If was between HCN1 and HCN2, whereas If from control mice activated slightly faster than expressed mHCN4 current (Figure 2C and Supplementary Figure S6). The voltage-dependence of activation, on the other hand, did not differ between HCN4-knockout and control cells (Figure 2D). Neither the voltages of half-maximal activation with −91.2±6.4 mV versus −90.4±3.4 mV (control, n=12 versus knockout, n=9, respectively) nor the slope factors (10.5 versus 11.2) differed significantly from one another. As HCN1 is activated at ∼20 mV more positive activation potentials (Wainger et al, 2001), this suggests that HCN1 is probably not a major contributor to If in controls or HCN4-knockouts. Taken together with the in situ hybridization and immunohistochemistry data described above, these results strongly indicate that sinoatrial If is mainly generated by HCN4. The remaining fraction in mice (∼25% of total If) is generated by HCN2 and, possibly to a lesser degree, by HCN1 subunits. The results are in accordance with the analysis of sinoatrial If in HCN2-deficient mice (Ludwig et al, 2003), where a ∼30% reduction of If in sinoatrial cells was found and where this residual If closely resembled an HCN4 current.
Figure 2.
Analysis of If in isolated knockout (L1) sinoatrial node cells. (A) Example traces of If recorded from a control (Ctr) and knockout (KO=L1) sinoatrial cell. Currents were activated by stepping from a holding potential of −40 mV to test potentials ranging from −130 mV to −20 mV for 8 s (in 10 mV steps), followed by −130 mV for 2 s before stepping back to the holding potential (protocol above current traces). Cells were held at −40 mV for 30 s before applying each new activation. Control (Ctr): HCN4L2/+, CAGGCre-ER™tg/0, 4–6 weeks after Tam; knockout (KO): HCN4L1/L2, CAGGCre-ER™tg/0, 4–6 weeks after Tam. (B) Means of voltage-dependent current densities from control (white symbols) and HCN4-knockout cells (black symbols) at activation potentials from −130 to −80 mV. The current density of the knockout cells (n=23) was reduced by 75% on average compared to control cells (n=32). The absolute current amplitudes showed equal percentages of reduction, see Supplementary Figure S6. (C) Time constants of activation (τact) at −100 mV of control and knockout cells compared to τact of the murine HCN channel isoforms 1, 2 and 4 expressed in HEK293 cells. Please refer to Supplementary Figure S6 for the analysis over the full range of activation potentials. The activation kinetic of If in knockout cells (black column) is between the ones of HCN1 and HCN2, whereas control cells (white column) are close to HCN4. (D) Means of normalized If from control (white symbols) and HCN4-knockout cells (black symbols) at activation potentials from −130 to −30 mV. Boltzman fits of the means are used to display the voltage-dependent activation curves. There are no significant differences between the curves.
Lack of HCN4 leads to recurrent pauses in sinoatrial node activity
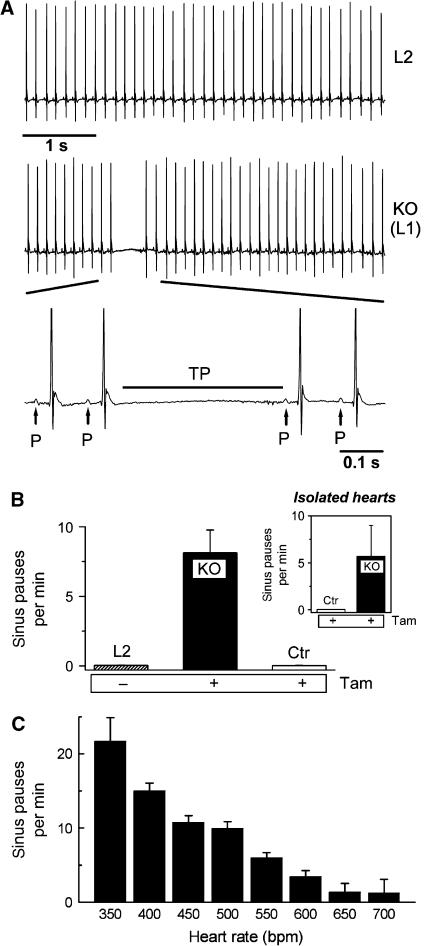
To assess the in vivo effect of deleting HCN4, we recorded electrocardiograms (ECGs) by telemetry continuously before and after gene deletion in the same animals. Around 3–6 days after administration of Tam, sinus pauses appeared which lasted between 200 and 500 ms (321±25 ms, n=8 mice, Figure 3A). In these periods, no electrical cardiac activity was observed (Figure 3A, bottom). The number of sinus pauses increased progressively over several days and reached 8.1±1.6 (n=8) per minute (Figure 3B). In contrast, no such sinus pauses were detected in the same mice before the injection of Tam (Figure 3B). Similarly, Tam-injected control mice (CAGGCre-ER™tg/0, HCN4L2/+) showed no sinus pauses indicating that Tam per se does not cause this arrhythmia. Long-term ECG recordings with simultaneous activity recordings revealed that the frequency of pauses depended on the activity state of the animals. There were significantly less pauses when the mice were highly active (Supplementary Figure S7). During high activity, the heart rates of the animals are usually much higher and reach up to 700 b.p.m. Thus, this ‘activity' dependence appears to be a ‘heart rate' dependence (Figure 3C). By far the highest number of sinus pauses occurred at resting heart rates of 300–450 b.p.m.
Figure 3.
HCN4-knockout mice show recurrent sinus pauses. (A) The ECG of the same mouse before (L2) and after (L1 or KO) Tam injection demonstrates a typical sinus pause occurring after deletion of HCN4. During the pauses, no P-waves are detected (bottom, enlarged ECG segment); the pauses are characterized by lack of electrical activity ranging from the end of the preceding T-wave to the beginning of the next regular P-wave (TP). (B) Quantitative analysis of the number of sinus pauses. L2 and KO represent the same animals before and after Tam injection, Control (Ctr) indicates HCN4L2/+ animals after Tam injection (n=8 for each group). (Inset) Isolated spontaneously contracting hearts from knockout, but not control animals display similar sinus pauses (n=4 for each group). (C) Mean sinus pause frequency plotted against the heart rate. Uninterrupted recordings over 7 days were used for this analysis. Heart rates were grouped into 50 b.p.m. intervals, numbers indicate the upper range limit (e.g. 350 refers to the interval of 300–350 b.p.m.).
Irrespective of the sinus pauses, the natural circadian rhythm concerning activity and heart rate was preserved in the HCN4-knockout mice. Furthermore, the mean heart rates, excluding the sinus pauses from the calculations, did not differ significantly (Supplementary Figure S7). In addition, no significant differences in other ECG parameters, namely conduction intervals (PQ, QRS) and repolarization intervals were detected in double mutant mice before and after Tam (Supplementary Table 2).
The sinus pauses in HCN4-knockouts could be caused by an arrest of sinoatrial impulse generation (sinus arrest) or by block of impulse conduction from the pacemaker center to the surrounding atrial tissue (exit block). Comparing the lengths of the sinus pauses with the RR cycle lengths immediately preceding and following the pause suggests sinus arrest as the pauses were not integer multiples of the RR interval (data not shown), but irregular with relative lengths of 1.7–6.1 multiples of the RR intervals.
Next, we investigated if the arrhythmia is indeed cardiac autonomous by recording ECGs from isolated spontaneously contracting hearts. We detected the same type of arrhythmia as observed in the in vivo recordings in isolated HCN4 mutant hearts (Figure 3B, inset). As expected, hearts from Tam-injected control mice did not display this phenotype.
HCN4 is not required for sympathetic upregulation of the heart rate
If is perhaps most widely known for its proposed role in the beta-adrenergic receptor-induced acceleration of cardiac pacemaker activity (Brown et al, 1979; DiFrancesco and Tortora, 1991; DiFrancesco, 1993; Zagotta et al, 2003; Cohen and Robinson, 2006). However, this role has been discussed controversially. We therefore examined the effect of beta-adrenergic agonists on heart rate regulation in adult HCN4-knockout animals.
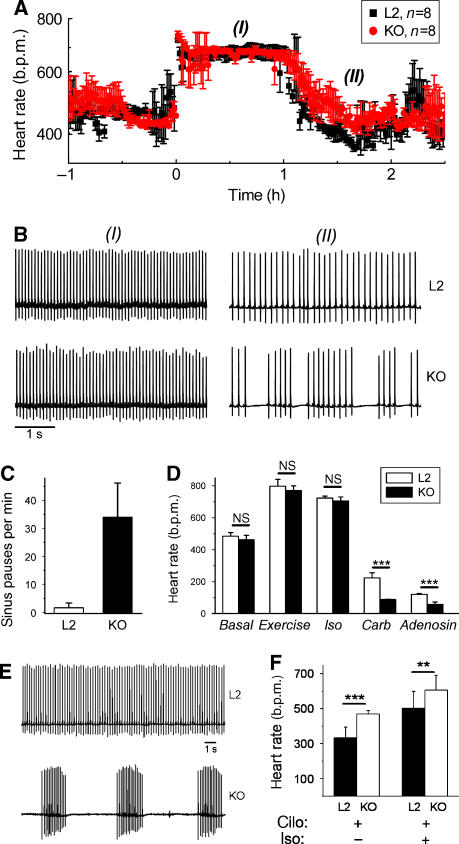
As described above, we found only very few sinus pauses when the mice were spontaneously highly active and had an accelerated heart rate. Even more surprisingly, we found no difference in heart-rate acceleration induced by the beta-agonist isoproterenol (0.5 mg/kg i.p.) between controls and mutants. Isoproterenol increased the heart rate in L2 (before Tam) and L1 (after Tam) mice to the same maximum level (L2: 724±12 b.p.m., n=8; L1: 705±26 b.p.m., n=8, Figure 4A and D). Heart rate increase by isoproterenol occurred at the same rate and lasted equally long in both groups (Figure 4A). The basal heart rate in both groups was not different (Figure 4D). The dose–response curves of isoproterenol did not differ significantly (Supplementary Figure S8) highlighting the undisturbed ability of HCN4-deficient mice to upregulate their heart rate. We also used a nonpharmacological protocol to stimulate the heart rate. Mice bearing radiotelemetry transmitters were trained to run on treadmill employing an exercise protocol. Similar to the results with isoproterenol, the increase in heart rate obtained using the exercise protocol was not different between control and knockout animals (Figure 4D).
Figure 4.
HCN4 is not required for upregulation of the heart rate. (A) Isoproterenol (0.5 mg/kg) injected at t=0 increased the heart rate of control (L2, n=8) and knockout (L1 or KO, n=8) animals to similar maximum levels. (B) Example ECG traces from control and knockout mice at (I), 0.5 h and (II), 1.5 h after isoproterenol injection. All traces are displayed at the same scale. See Supplementary Figures S5 and S6 for enlarged ECGs. (C) Quantitative analysis of the sinus pauses in phase (II). The number of sinus pauses increased fourfold compared to the basal level (Figure 3B). (D) Mean heart rates of control and knockout mice during stimulation by an exercise protocol (running on a treadmill) or isoproterenol (iso, 0.5 mg/kg) did not differ significantly. In contrast, carbachol (carb, 0.5 mg/kg) and the A1 adenosine receptor agonist CCPA (adenosine, 0.3 mg/kg) induced highly significant lower heart rates in knockouts (n=8 for both genotypes, ***P<0.001). (E) Example ECG traces recorded 30 min after the injection of 0.5 mg/kg cilobradine of the same mouse before (L2) and after (KO) Tam injection. Both traces are displayed at the same scale (bar=1 s). (F) Analysis of heart rates (n=8 mice) reveals a changed effect of cilobradine in knockouts. The decrease of the heart rate by cilobradine (excluding sinus pauses in the calculation) is less in knockout than in control animals. Additional stimulation by isoproterenol leads to a similar heart rate increase in both groups. cilo, cilobradine; iso, isoproterenol. ***P<0.001; **P<0.01.
In addition, as mentioned above, the phosphorylation status of Phospholamban in SAN extracts (Supplementary Figure S3) did not differ between control and mutants indicating similar basal cAMP levels in sinoatrial cells of both groups (Vinogradova et al, 2006).
Enhanced sinoatrial dysfunction in the transition phase from stimulated to basal conditions
Remarkably, as just described, we detected no arrhythmic episodes in knockout mice during the maximum stimulation phase (Figure 4B, (I) and Supplementary Figure S9). However, sinus pauses appeared strikingly numerous in these mice when the heart rate returned to the basal level (Figure 4B (II); for an enlargement of the traces see Supplementary Figure S10). In this period, knockout animals displayed 34.0±12.1 (n=8) pauses per minute representing a fourfold increase compared to basal level (Figure 4C). Furthermore, we analyzed heart rate modulation by two other pathways and found that mutant mice were also not impaired in heart-rate decrease in response to the muscarinic agonist carbachol or the A1 adenosine receptor agonist CCPA (Wickman et al, 1998) (Figure 4D). Instead, the mutant mice showed a significantly increased response to these two substances and reached heart rates as low as 121±5 and 89±2 bmp (n=8, P<0.05) after carbachol and CCPA, respectively (Figure 4D). Longer sinus pauses ranging from 1 to 2 s were observed (data not shown) underscoring an enhanced sinoatrial node dysfunction under these conditions.
Fast heart rates in HCN4-knockout mice are still possible when the residual If is pharmacologically targeted
To examine whether the residual If could mediate the heart rate acceleration in HCN4-knockout mice, we used the If inhibitor cilobradine to maximally reduce any If in vivo. Cilobradine belongs to the group of the so-called ‘sinus node inhibitors' including, for example, ivabradine. These sinus node inhibitors effectively block all four HCN channel subtypes and induce a dose-dependent bradycardia in animals and humans (DiFrancesco and Camm, 2004; Stieber et al, 2006). Interestingly, cilobradine did not steadily reduce the heart rate in HCN4-knockout mice, but aggravated the dysrhythmic patterns. The main effect after injection of 0.5 mg/kg of cilobradine, which caused a moderate and rhythmic bradycardia in control mice, was a striking increase of the sinus pause durations of to up to 10 s (Figure 4E). Nonetheless, the mean heart rate in between these pauses was even faster than in control mice (Figure 4F). Higher doses of cilobradine up to 2 mg/kg had comparable effects in the mutants, that is, increasing prolongation of individual sinus pauses but normal heart beats in between. Beta-adrenergic stimulation by isoproterenol after the pretreatment with 0.5 mg/kg cilobradine was still able to accelerate the heart rate to a mean of 605±85 b.p.m. (Figure 4F). However, the sinus pauses were not completely ablated in this situation, in contrast to beta-adrenergic stimulation in the absence of If blockers as shown above. ECG complexes were normal in all cases (Supplementary Figure S11). These experiments further support the finding that If is important for maintaining a rhythmic basic heart rate, but not for the acceleration of the heart rate.
Beta-adrenergic stimulation of isolated SAN cells
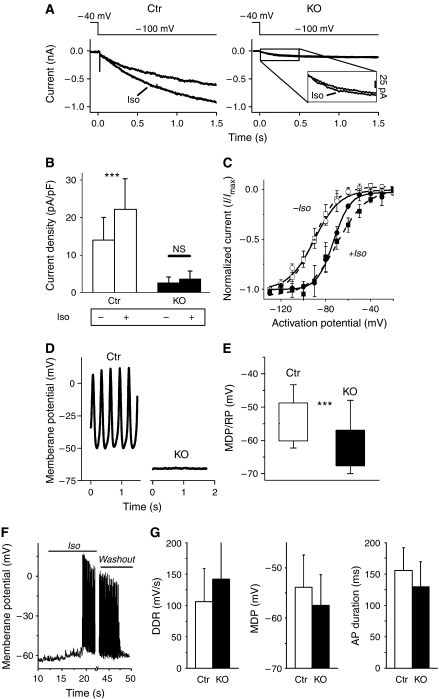
Finally, we investigated the effect of beta-adrenergic stimulation on electrophysiological properties on the single cell level. First, we examined the modulation of If by beta-adrenergic agonists in isolated sinoatrial cells. As expected (DiFrancesco and Tortora, 1991; Mangoni and Nargeot, 2001), superfusion with isoproterenol significantly increased the amplitude of If in wild-type SAN cells (n=14, P<0.05, Figure 5A and B) and shifted the voltage-dependent activation curve towards more positive activation potentials by ∼20 mV (Figure 5C). The slope factor decreased, from 9.92±1.88 to 7.01±1.07, resulting in a steeper voltage dependence of activation. In comparison, the activation curve of the residual If in HCN4-knockout cells also became steeper with a slope factor of 7.66±1.06 versus 11.16±3.51 before the stimulation. In addition, the activation curve was significantly shifted to the right, even though the extent of the shift was only ∼15 mV.
Figure 5.
Isoproterenol does not significantly increase the residual If in HCN4-deficient sinoatrial cells, but induces the spontaneous discharge of action potentials. (A) Examples of If traces elicited at −100 mV from control (left panel) and knockout (right panel) SAN cells before and after application of 1 μM isoproterenol (iso). An enlargement of If in HCN4-knockout cells is shown in the inset (right panel). (B) If in knockout cells (black columns, n=15) was only slightly modulated by isoproterenol resulting in a tendency towards an increased current density after 0.5 s (P=0.057). In contrast, If in control cells (white columns, n=14) was readily accelerated resulting in a significant current increase after 0.5 s (P<0.05). (C) Voltage-dependent activation curves of both control (squares) and HCN4-knockout (circles) If are shifted towards more-positive activation potentials by 1 μM isoproterenol (+iso, filled symbols; −iso, open symbols). V1/2 and slope factors were calculated using the Boltzman fit of the means, here displayed as open and broken lines. (D–G) Membrane potential recordings from isolated sinoatrial node cells. MDP/RP refers to the maximum diastolic potential (MDP) of spontaneously firing cells and the resting membrane potential (RP) of silent cells, respectively. (D) Example traces of membrane potential recordings from control and knockout cells. Typical spontaneous SAN action potentials were readily recorded from control cells (11 out of 13 cells), but rarely from knockout cells (3 out of 27 cells). The majority of these cells (24/27) were electrically silent. (E) Analysis of membrane potentials revealed a significant more negative membrane potential of knockout cells. Boxes indicate mean±s.d. of all measured cells (27 KO, 13 Ctr cells), error bars represent the absolute range. ***P<0.001. (F) Example trace of a membrane potential recording from an HCN4-deficient SAN cell that was ‘silent' at first. Superfusion with 1 μM isoproterenol (‘iso') induced a spontaneous discharge of action potentials. After 30 s, superfusion was switched back to iso-free bath solution (‘washout'), which terminated the firing after about 20 s. (G) Action potential parameters of iso-stimulated knockout cells (black columns, n=7) were not significantly different from control pacemaker cells. In addition to the parameters displayed here, no significant differences were also found with upstroke velocity, overshoot potential and repolarization velocity.
However, the absolute and capacity-corrected amplitude of the small residual If in knockout cells was only marginally enhanced, resulting in a nonsignificant tendency towards a current amplitude increase (n=15, P=0.057). Application of the membrane-permeable cAMP analogon 8-Br-cAMP had a similar effect on the If in HCN4-knockout cells as isoproterenol, that is, the current increased from 2.6±2.1 to 4.4±2.9 pA/pF (n=6).
Isolated sinoatrial cells lacking HCN4 do not fire properly but can be stimulated to do so
Next, we analyzed the ability of isolated sinoatrial node cells of control and HCN4-knockout mice to generate pacemaker action potentials, both without and with beta-adrenergic stimulation. Under control conditions, typical spontaneous SAN action potentials were readily recorded from control cells (11 out of 13 cells). However, around 90% (24 out of 27) of mutant sinoatrial cells did not fire spontaneously and showed a stable resting membrane potential under these conditions (Figure 5D). Analysis of the maximum diastolic potential and the resting membrane potential, respectively, showed that mutant cells are hyperpolarized by about −8 mV compared to control cells (P<0.05, Figure 5E).
Superfusion of the cells with isoproterenol raised the membrane potential within several seconds towards the threshold potential and spontaneous action potentials could be recorded from HCN4-knockout cells thereafter (Figure 5F). In three out of seven cells, the spontaneous regular discharge of action potentials continued as long as the superfusion with isoproterenol lasted, whereas in the other cells the firing tended to get irregular after several seconds, but did not cease altogether. The characteristics of the individual action potentials were similar to the control action potentials shown in Figure 5D. The MDP of the knockout cells was still slightly more negative compared to control cells (−57.5±6 versus −53.9±6.5), but all parameters analyzed including diastolic depolarization rate, action potential duration, upstroke velocity, overshoot potential and repolarization velocity were not significantly different (Figure 5G). These results suggest that, even though isolated HCN4-knockout cells are able to generate pacemaker action potentials under certain conditions, repolarizing influences are imperfectly counterbalanced.
Discussion
Our results challenge previous assumptions about the physiological role of If in cardiac pacemaking (Brown et al, 1979; DiFrancesco and Tortora, 1991; DiFrancesco, 1993; Zagotta et al, 2003; Cohen and Robinson, 2006). We find that a large part of this current, namely the fraction carried by HCN4 (∼75% of total If), is not required to mediate the acceleration of the heart rate in response to beta-adrenergic stimulation. Our experiments cannot completely exclude the possibility that the residual If remaining after deletion of HCN4 contributes to this role. However, the remaining If in the mutants accounted for only about 25% of wild-type If and we found no evidence for an increased expression of other HCN subunits in the mutants, which may be expected if HCN channels are indeed vital for the proper generation of a fast cardiac rhythm.
Allowedly, similarly to control cells, the activation curve of the residual If in HCN4-knockout sinoatrial cells was rightward shifted by beta-adrenergic stimulation and became steeper, so that small changes of the membrane potential had larger effects on the current amplitude. Generally, a rightward shift results in channel activation at more positive membrane potentials (closer to the threshold potential), resulting in an increased ion flow at a given potential within the dynamic activation range. This is usually viewed in association with enhanced pacemaking, that is, an accelerated slow depolarization rate and consequently a faster heart rate. However, to calculate the voltage dependence, the currents relative to the maximum current of each cell are used. In our case, this shift in voltage dependence did not lead to a significant increase of the absolute amount of current. Even though it is not exactly known how much (If−) current is needed to significantly enhance the slow depolarization rate, it seems unlikely that this small current increase could be solely responsible for the observed increase in heart rate of mutants following beta-adrenergic stimulation.
Moreover, we found that acceleration of the heart rate was still possible when both HCN4 was genetically deleted and the residual If was pharmacologically inhibited by an If-blocker. This is in accordance with our previous finding that, although cilobradine reduces the heart rate in wild-type mice, this If inhibitor does not prevent the increase of the heart rate upon sympathetic stimulation. In particular, the proportional increase of the heart rate was the same in cilobradine-pretreated animals as in placebo-pretreated controls after injection of isoproterenol (Stieber et al, 2006). This strongly supports our idea that stimulation of the heart rate is independent of If.
The fact that isoproterenol is able to stimulate the spontaneous discharge of regular sinoatrial pacemaker action potentials in isolated sinoatrial cells from HCN4-knockout mice does not contradict this suggestion. Rather, this supports findings by other groups postulating newly discovered mechanisms to be responsible for the PKA-dependent regulation of the heart rate, such as intracellular calcium cycling involving L-type calcium channels, Ryr2 receptors and the sodium-calcium exchanger NCX1 (Vinogradova et al, 2005, 2006). These channels and transporters have not been targeted by the deletion of HCN4, thus they should be functionally intact in HCN4-knockout mice.
Nevertheless, the results obtained from isolated SAN cells indicate an important role for HCN4 as a determinant of the membrane potential in vivo and in vitro. Deletion of the channel shifts the membrane potential in isolated cells under basal conditions to a range where spontaneous firing is mostly abolished. Under the influence of beta-adrenergic stimulation, however, the cells were able to generate spontaneous action potentials. This is in agreement with the observation that there were no disturbances in the cardiac rhythm in HCN4-knockout mice under beta-adrenergic stimulation, but an increased number of stops in cardiac pacemaking at low heart rates.
It does not fully explain why cardiac pacemaking in vivo stopped only sporadically, but difference in the spontaneous activity of isolated sinoatrial cells versus the complete SAN/heart may be explained by differing levels of cyclic nucleotides or network properties of the intact tissue: in the SAN, there might be one pacemaker cell at a given time point that functions as the ‘leading' pacemaker cell. In case this cell stops generating action potentials, another cell might take over. Only if there is a considerable amount of ‘silent' cells present at the same time, pacemaking is interrupted until one cell takes the leading position again. There is the possibility that other cell types such as atrial cardiomyocytes started to initiate the heart beat after deletion of HCN4 in the SAN. This, however, seems unlikely as unaltered P-waves and PQ-durations were measured in the knockout animals demonstrating that cardiac excitation continued to start spreading from the same area of the atria as before the deletion of HCN4.
Besides the considerations about the mechanisms underlying sympathetic heart rate stimulation, there was another unexpected finding of our study. Several studies have detected mutations of the HCN4 gene in humans resulting in the generation of an If with changed parameters associated with bradycardia as well as other cardiac arrhythmias (Schulze-Bahr et al, 2003; Ueda et al, 2004; Milanesi et al, 2006). In addition, the sinus node inhibitor ivabradine, a substance similar to cilobradine, lowers the heart rate of animals and humans, assumingly by inhibiting If in SAN cells (DiFrancesco and Camm, 2004; Stieber et al, 2006). In contrast to these findings, HCN4-knockout mice do not show a slower heart rate than control mice, despite the strong reduction of If. There could be several reasons for this disagreement: first, the HCN4 mutations detected in human patients are heterozygous mutations and no deletions, that is, there is still one intact allele. If, or rather HCN4 currents in these patients are therefore not deleted, but differently modulated, which could result in quite different effects than a complete lack of the current. Second, our study was conducted in mice. Even though the expression pattern of HCN channels, especially the high expression of HCN4 in the sinoatrial node seems to be similar in all species, the functional importance of HCN4 might be different. At low heart rates, the deletion of HCN4 resulted in a striking arrhythmic pattern, which disappeared at faster heart rates. Compared to small animals, humans have a 10-times-slower basic heart rate, which under beta-adrenergic stimulation just reaches the ‘slow' heart rates observed in mice. Third, sinus node inhibitors lower dose-dependently the heart rate in humans as well as in wild-type mice. However, it has been demonstrated that these drugs also block channels and targets independent of If (Bois et al, 1996; Chevaleyre and Castillo, 2002), and extra care in interpreting data using these pharmacological tools to analyze the role of If has been advised. Provided that indeed the inhibition of HCN channels, and not other ion channels, is responsible for the decrease in heart rate by these drugs, there is clearly a difference in the genetic deletion of HCN4 versus the sudden reduction of If mediated by a partial block of all HCN subtypes. The genetic deletion takes several days and finally results in an irreversible ablation of If-current. This provides time and may create a greater ‘driving force' for the system to adjust to another pacemaking mechanism that is available.
Conclusion: a model for the role of If in cardiac pacemaking
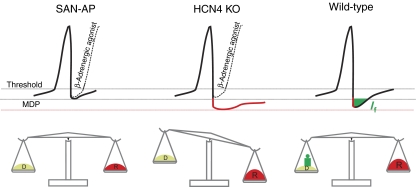
Even though the main purpose of this study was to define the role of HCN4 in the cardiac pacemaking process, we finally suggest a model for the role of If in cardiac pacemaking (Figure 6). Whereas other HCN subunits also contribute, HCN4 undoubtedly underlies the main proportion of sinoatrial If. Based on the results from this study using a temporally controlled knockout of HCN4, we propose that If largely functions as a backup mechanism, which guarantees stable pacemaking during conditions where repolarizing currents are increased (e.g. muscarinic stimulation or transition from activated to basal cardiac state). In these situations, HCN4 channels provide an inward depolarizing current keeping the dynamic relationship between depolarizing and repolarizing currents well balanced. Hence, the channels provide a ‘depolarization reserve', ensuring stable spontaneous firing, driving the heart under every physiological condition.
Figure 6.
Proposed role for HCN4 channels in sinoatrial node cells. top, Spontaneous action potentials under basal (solid line) and beta-adrenergic-stimulated (broken line) conditions. MDP, maximum diastolic potential. Lower part, scale illustrating the overall relationship between depolarizing and repolarizing currents. Left, regular SAN action potential (SAN-AP). To ensure stable pacemaking over time, depolarizing currents are in a dynamic balance with repolarizing currents (indicated by balanced scale). In most situations, and particularly during sympathetic stimulation, HCN4 is not required to promote stable pacemaking. Middle and right, SAN action potentials after an increase in repolarizing currents (e.g. muscarinic stimulation or transition from activated to basal cardiac state; symbolized by red weight). In wild-type cells (right), HCN4 is activated and provides a depolarizing current (green) keeping the system well-balanced. In HCN4-deficient cells (middle), the membrane potential tends to remain sub-threshold fostering the appearance of sinus pauses.
Contrary to expectations, we find that HCN4 channels do not seem to be required to mediate the beta-adrenergic-dependent modulation of the heart rate in mice. Other proteins, such as ryanodine receptors, L-type calcium channels or as yet unidentified mechanisms may be involved in this signal transduction pathway. In conclusion, our study suggests that the HCN4 channel is not critical for proper acceleration of the heart rate; however, the channel is necessary for pacemaking in physiological situations where a delay in rhythm generation is imminent.
Materials and methods
All animal studies were in compliance with the Guide for the Care and Use of Laboratory Animals as published by the US National Institutes of Health and were approved by the local regulatory authority (Regierung von Oberbayern).
Generation of inducible HCN4-knockout mice
HCN4+/L1 mice were crossed with transgenic CAGGCre-ER™ animals to obtain double heterozygous mice (HCN4+/L1, CAGGCre-ER™tg/0). These mice were mated with animals homozygous for the floxed HCN4 locus (HCN4L2/L2) to generate offspring (HCN4L2/L1, CAGGCre-ER™tg/0) that allow Tam-inducible deletion of HCN4. Cre-mediated recombination was induced by i.p. administration of Tam (6 mg/25 g bodyweight, Sigma) for 3 consecutive days. Tam was freshly dissolved in miglyol oil (Caelo) at a concentration of 20 mg/ml. Experiments with Tam-treated animals were performed at least 4 weeks after the injection unless otherwise mentioned in the text.
Isolation of sinoatrial node tissue
Mice were killed by cervical dislocation. Hearts were quickly removed and placed in ice-cold PBS, pH 7.4, 4 mM EDTA. Right atrium including superior vena cava was isolated under a dissecting microscope and cleared of connective tissue and fat. For immunohistochemistry, tissue was fixed in 2% paraformaldehyde for 2 h at room temperature. For RNA and protein isolation, the SAN region (approximately 1 × 1 mm), limited by crista terminalis, atrial septum and orifice of superior vena cava was carefully dissected and flash-frozen in liquid nitrogen.
RT–PCR and Western blot
SAN tissue was pulverized under liquid nitrogen. Total RNA was isolated using RNeasy Fibrous Tissue Micro Kit (QIAGEN, Hilden, Germany) and cDNA was amplified using Superscript II (Invitrogen) or a OneStep RT–PCR Kit (QIAGEN, Hilden, Germany). Primer pairs for amplification of HCN1–4 and GAPDH were intron-spanning to preclude amplification of genomic DNA. For Western analysis, pulverized tissue was boiled in 2% SDS, 50 mM Tris (pH 7.4), separated by 7.5% SDS–PAGE, blotted on a PVDF membrane (Millipore) and probed with an HCN4-specific antibody (Stieber et al, 2003). Equal protein loading was ascertained by the use of a GAPDH antibody (Santa Cruz). Bound antibodies were visualized by the ECL system (NEN). Two to three sinoatrial nodes were pooled for protein and RNA isolation.
Quantitative RT–PCR
One-tube RT–PCR was performed using a Quantitect Probe RT–PCR Kit (QIAGEN, Hilden, Germany). Expression of genes was determined by TaqMan assays on an ABI Prism 7900. Gene specific primers and probes were purchased from Applied Biosystems (Darmstadt, Germany). For each RT–PCR, the threshold cycle (CT) defined as the cycle at which the fluorescence exceeds 10 times the standard deviation of the mean baseline emission for cycles 3–10 was determined. The CT value of each gene was normalized to β-actin according to the following formula: ΔCT=CT(examined gene)−CT(β-actin). ΔCT values of controls were then compared to knockouts. Genes with CT>32 were not included in the final analysis. TaqMan assays used are given in Supplementary data.
Immunohistochemistry
Fixed tissue was embedded in paraffin and cut into 10 μm sections. Endogenous peroxidase activity was quenched and antigen retrieval was performed as described previously (Stieber et al, 2003). Sections were incubated with HCN4 (Stieber et al, 2003) or β-galactosidase antibody (ICN Biomedicals). Bound antibodies were detected using Vectastain elite ABC kit (Vector) and 3,3′-diaminobenzidinetetrahydrochloride.
Electrophysiology
Isolation of SAN cells was basically performed as described previously (Stieber et al, 2006). Briefly, the excised SAN area was enzymatically digested using collagenase B and elastase (Roche, Mannheim, Germany) and single SAN cells were released from the tissue by agitation. Cells were stored in a high potassium storage solution at 4°C for at least 3 h before electrophysiological recordings. If from SAN and transfected HEK293 cells was recorded with the whole-cell patch clamp recording technique at a temperature of 22±2°C. Membrane and action potentials from SAN cells were recorded at 36±1°C using the perforated patch technique with 200 μg/ml Amphotericin B added to the intracellular solution. Isoproterenol or 8-Br-cAMP were dissolved in bath solution and applied to the cells via a superfusion system. Composition of all solutions and calculation of If and AP parameters are described in Supplementary data.
ECG recordings in unrestrained mice and from isolated hearts
Telemetric ECG recordings were performed as described (Ludwig et al, 2003) with radiotransmitters (DSI, St Paul, USA) implanted intraperitoneally. Drugs were injected i.p. For forced physical activity, animals were trained to run on a custom made, electrically driven treadmill at a speed of 0.4 m/s and 20% ascending slope. To record ECGs from isolated hearts, the excised hearts were placed in 37°C warmed Tyrode solution aerated with carbogen (O2/CO2 95%/5%). Hearts were perfused retrograde with Tyrode solution through the aorta and ECG electrodes were placed on right atrium and apex of the heart. Evaluation of the cardiac rhythm was done after an equilibration period (stable ECG signals for at least 30 min).
Statistical analysis
All values given are mean±s.d. Differences between groups were analyzed by Student's t-test.
Supplementary Material
Supplementary Information
Acknowledgments
We thank A Vens, B Layh and S Gabriel for excellent technical support and A Pahl for advice on quantitative PCR. We thank Dr J Molkentin (Children's Hospital Medical Center, Cincinnati, USA), Dr L DeWindt (Interuniversity Cardiology Institute, Utrecht, the Netherlands), Dr A Berns (Netherlands Cancer Institute, Amsterdam, the Netherlands), Dr M Schneider (Baylor College of Medicine, Houston, USA), Dr R Kahn (Joslin Diabetes Center, Boston, USA), Dr J Bruening (University of Cologne, Germany), and Dr P Savatier (Ecole Normale Superieure, Lyon, France) for providing materials. The research was supported by grants from Deutsche Forschungsgemeinschaft to AL and FH.
References
- Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A (1997) Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res 25: 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Schneider A, Wahl C (2002) Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med 12: 206–212 [DOI] [PubMed] [Google Scholar]
- Bois P, Bescond J, Renaudon B, Lenfant J (1996) Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 118: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HF, DiFrancesco D, Noble SJ (1979) How does adrenaline accelerate the heart? Nature 280: 235–236 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE (2002) Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci USA 99: 9538–9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IS, Robinson RB (2006) Pacemaker current and automatic rhythms: toward a molecular understanding. Handb Exp Pharmacol 171: 41–71 [DOI] [PubMed] [Google Scholar]
- Dibb KM, Rose T, Makary SY, Claydon TW, Enkvetchakul D, Leach R, Nichols CG, Boyett MR (2003) Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K(+) channel. J Biol Chem 278: 49537–49548 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D (1993) Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55: 455–472 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Camm JA (2004) Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs 64: 1757–1765 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P (1991) Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351: 145–147 [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318 [DOI] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W (1993) Cardiac pacemaking in the sinoatrial node. Physiol Rev 73: 197–227 [DOI] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Xie LH, Noma A, Ohmori H (1999) Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem 274: 12835–12839 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R (2001) Molecular diversity of pacemaker ion channels. Annu Rev Physiol 63: 235–257 [DOI] [PubMed] [Google Scholar]
- Lei M, Honjo H, Kodama I, Boyett MR (2001) Heterogeneous expression of the delayed-rectifier K+ currents i(K,r) and i(K,s) in rabbit sinoatrial node cells. J Physiol 535: 703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M (2007) Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res 73: 729–738 [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22: 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Stieber J, Moosmang S, Herrmann S, Biel M, Hofmann F (2004) HCN channels: from genes to function. In: Cardiac Electrophysiology: From Cell to Bedside, Zipes DP, Jalife J (eds), pp 59–65. Philadelphia: Saunders [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M (1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587–591 [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J (2006) Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol 90: 38–63 [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J (2001) Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res 52: 51–64 [DOI] [PubMed] [Google Scholar]
- Miake J, Marban E, Nuss HB (2002) Biological pacemaker created by gene transfer. Nature 419: 132–133 [DOI] [PubMed] [Google Scholar]
- Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D (2006) Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354: 151–157 [DOI] [PubMed] [Google Scholar]
- Minamino T, Gaussin V, DeMayo FJ, Schneider MD (2001) Inducible gene targeting in postnatal myocardium by cardiac-specific expression of a hormone-activated Cre fusion protein. Circ Res 88: 587–592 [DOI] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268: 1646–1652 [DOI] [PubMed] [Google Scholar]
- Noma A (1996) Ionic mechanisms of the cardiac pacemaker potential. Jpn Heart J 37: 673–682 [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480 [DOI] [PubMed] [Google Scholar]
- Schram G, Pourrier M, Melnyk P, Nattel S (2002) Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res 90: 939–950 [DOI] [PubMed] [Google Scholar]
- Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D (2003) Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 111: 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS (1999) Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res 85: e1–e6 [DOI] [PubMed] [Google Scholar]
- Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25 [DOI] [PubMed] [Google Scholar]
- Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100: 15235–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J, Stockl G, Herrmann S, Hassfurth B, Hofmann F (2005) Functional expression of the human HCN3 channel. J Biol Chem 280: 34635–34643 [DOI] [PubMed] [Google Scholar]
- Stieber J, Wieland K, Stockl G, Ludwig A, Hofmann F (2006) Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol 69: 1328–1337 [DOI] [PubMed] [Google Scholar]
- Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y, Yasunami M, Takishita S, Yamashina A, Ohe T, Sunamori M, Hiraoka M, Kimura A (2004) Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem 279: 27194–27198 [DOI] [PubMed] [Google Scholar]
- Vallier L, Mancip J, Markossian S, Lukaszewicz A, Dehay C, Metzger D, Chambon P, Samarut J, Savatier P (2001) An efficient system for conditional gene expression in embryonic stem cells and in their in vitro and in vivo differentiated derivatives. Proc Natl Acad Sci USA 98: 2467–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M (1995) The pacemaker current (I(f)) does not play an important role in regulating SA node pacemaker activity. Cardiovasc Res 30: 309–310 [PubMed] [Google Scholar]
- Verheijck EE, Wilders R, Bouman LN (2002) Atrio-sinus interaction demonstrated by blockade of the rapid delayed rectifier current. Circulation 105: 880–885 [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG (2006) High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514 [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Maltsev VA, Bogdanov KY, Lyashkov AE, Lakatta EG (2005) Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann N Y Acad Sci 1047: 138–156 [DOI] [PubMed] [Google Scholar]
- Vooijs M, Jonkers J, Berns A (2001) A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2: 292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411: 805–810 [DOI] [PubMed] [Google Scholar]
- Wickman K, Nemec J, Gendler SJ, Clapham DE (1998) Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20: 103–114 [DOI] [PubMed] [Google Scholar]
- Yu X, Duan KL, Shang CF, Yu HG, Zhou Z (2004) Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci USA 101: 1051–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425: 200–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information