Abstract
Rab guanosine triphosphatases regulate intracellular membrane traffic by binding specific effector proteins. The yeast Rab Sec4p plays multiple roles in the polarized transport of post-Golgi vesicles to, and their subsequent fusion with, the plasma membrane, suggesting the involvement of several effectors. Yet, only one Sec4p effector has been documented to date: the exocyst protein Sec15p. The exocyst is an octameric protein complex required for tethering secretory vesicles, which is a prerequisite for membrane fusion. In this study, we describe the identification of a second Sec4p effector, Sro7p, which is a member of the lethal giant larvae tumor suppressor family. Sec4-GTP binds to Sro7p in cell extracts as well as to purified Sro7p, and the two proteins can be coimmunoprecipitated. Furthermore, we demonstrate the formation of a ternary complex of Sec4-GTP, Sro7p, and the t-SNARE Sec9p. Genetic data support our conclusion that Sro7p functions downstream of Sec4p and further imply that Sro7p and the exocyst share partially overlapping functions, possibly in SNARE regulation.
Introduction
Cell polarization is important for processes as diverse as cell movement, axonal outgrowth, secretion of hormones, and cell differentiation. Polarization requires the vectorial delivery of secretory vesicles to, and their subsequent fusion with, the plasma membrane. The yeast Saccharomyces cerevisiae is an excellent model organism to study mechanisms of polarization because it propagates through the polarized outgrowth of a bud.
In S. cerevisiae, the polarized transport of secretory vesicles to the bud tip or, later in the cell cycle, to the mother-bud neck, depends on the actin cytoskeleton and the actin-based myosin motor protein Myo2p (Pruyne et al., 1998; Karpova et al., 2000). An important factor in this transport event is Sec4p, the founding member of the Rab branch of the Ras superfamily of small GTPases (Salminen and Novick, 1987; Goud et al., 1988). Rab proteins are so-called molecular switches, which cycle between an “on” (GTP bound) and “off” (guanosine 5′-diphosphate [GDP] bound) state. This GTPase switch is influenced by guanine nucleotide exchange factors (GEFs), which trigger the binding of GTP, thus activating the Rabs, and GTPase-activating proteins, which accelerate hydrolysis of the bound GTP to GDP, inactivating the GTPase (for review see Pfeffer, 2001; Segev, 2001). Rabs are thought to accomplish their function by binding specific effector proteins in their GTP-bound state (for review see Pfeffer, 2001; Zerial and McBride, 2001).
Sec4p was found in a screen for mutants that block exocytosis and accumulate secretory vesicles at a restrictive temperature (Novick et al., 1980). Mutants of SEC2, the Sec4p GEF, show an accumulation of vesicles randomly distributed throughout the cell, implying that the activation of Sec4p by Sec2p directs the polarized delivery of secretory vesicles (Walch-Solimena et al., 1997). Supporting the view that activated GTP-Sec4p promotes Myo2p-dependent movement of secretory vesicles along actin cables, Sec4p was found to coimmunoprecipitate with Myo2p (Wagner et al., 2002).
Although mutations in SEC4 tightly block secretion (Novick et al., 1980), mutations in actin (ACT1) and MYO2 depolarize secretion and cell surface growth but do not block secretion (Govindan et al., 1995; Karpova et al., 2000). These results imply that Sec4p has at least one function in addition to its role in polarized vesicle delivery. A clue toward one such function came from the identification of Sec15p as a Sec4p effector (Guo et al., 1999b). Sec15p is a subunit of the exocyst, an octameric complex required for tethering secretory vesicles to the plasma membrane in preparation for fusion (TerBush et al., 1996; Guo et al., 1999a). Sec8p, another exocyst subunit, was recently found to coimmunoprecipitate with Sec4p, suggesting that the entire exocyst complex acts downstream of Sec4p (Toikkanen et al., 2003). Furthermore, Sec4p is required for the assembly of the exocyst complex (Guo et al., 1999b).
It has become increasingly clear that Rab GTPases interact with a variety of effectors in order to fulfill their functions in the cell (for review see Zerial and McBride, 2001; Deneka et al., 2003). Combined with the evidence that Sec4p regulates both the transport of secretory vesicles to and fusion with the plasma membrane, it seems likely that there are still more Sec4p effectors to be found. The identification of additional effectors would provide important new insights into the molecular function of Sec4p.
Lethal giant larvae (lgl) was first identified as a tumor suppressor gene in the fly Drosophila melanogaster (for review see Wodarz 2000; Bilder 2004). lgl mutant flies were found to develop malignant tumors in the larval brain and imaginal discs that appear to result from a loss of cell polarity. Subsequently, homologues of lgl have been identified in many organisms, ranging from yeast to humans (for review see Bilder 2004). Because lgl family members are often found to be associated with the actin cytoskeleton, it has been argued that the observed polarity defects in cells bearing lgl mutations are caused by defects in the actin cytoskeleton (Strand et al., 1994; Peng et al., 2000; for review see Baek, 2004). However, data are accumulating that lgl family members function in polarized membrane traffic. D. melanogaster lgl is required for the targeting of proteins to the basolateral membrane (Peng et al., 2000), and the yeast homologues of lgl, Sro7p, and Sro77p (also known as Sop1p and Sop2p, respectively) are redundantly required for exocytosis (Lehman et al., 1999). Sro7p and Sro77p are 55% identical (Kagami et al., 1998; Lehman et al., 1999). Both proteins were originally identified as high-copy suppressors of rho3Δ (Kagami et al., 1998), a Rho GTPase required for actin cytoskeleton polarity and polarized exocytosis (Matsui and Toh-e, 1992b; Imai et al., 1996; Adamo et al., 1999).
In this study, we describe the identification of Sro7p as an effector of Sec4p. Sro7p from yeast extracts as well as purified Sro7p interact specifically with the GTP-bound form of Sec4p. Sro7p was found to coimmunoprecipitate with Sec4p, demonstrating that the interaction we observed in vitro also occurs in vivo. Moreover, we found that Sro7p, Sec4p, and the t-SNARE Sec9p can form a ternary complex, suggesting that Sec4p regulates SNARE function through Sro7p. In agreement with this, genetic analysis shows that Sro7p shares a function with the exocyst downstream of Sec4p.
Results
Identification of Sec4p effectors
We used an affinity purification approach to identify potential Sec4p effectors. GST-tagged Sec4p was purified from bacteria (Fig. 1, A and B). GST-Sec4p bound to glutathione beads was loaded with either GTPγS or GDP and, subsequently, incubated with wild-type yeast extract. As a control, GST bound to beads was also incubated with extract. The beads were washed several times, and bound proteins were eluted with high salt (see Sec4p affinity chromatography for details). Despite the presence of background proteins (Fig. 1 C, GST lane), differences could be readily detected between the protein bands in the GST-Sec4p-GTPγS and GST-Sec4p-GDP affinity chromatography samples (Fig. 1 C). The protein mixture of each sample was TCA precipitated and subjected to mass spectrometry analysis. Common background proteins were identified by their abundance in both samples and by a survey of published literature (Ho et al., 2002). By definition, an effector binds more strongly to the GTP-bound form of a GTPase than to the GDP-bound form. One measure of the relative abundance of a protein in a protein mixture examined by mass spectrometry is the percentage of residues in the protein sequence that are represented by at least one peptide. We considered all proteins with an at least threefold higher coverage in the sample retrieved from the GTPγS-bound versus the GDP-bound form of Sec4p to be potential Sec4p effectors. Among the identified proteins meeting these criteria was Sro7p, a yeast member of the lgl family of proteins.
Figure 1.

Affinity purification of Sec4p-binding proteins. (A) GST-Sec4p (Sec4) and GST were purified from E. coli, subjected to SDS-PAGE electrophoresis, and visualized by Coomassie staining. (B) GST-Sec4p (Sec4) and GST were purified from E. coli and subjected to Western blot analysis using α-Sec4 antibody. (C) Glutathione beads coated with GST-Sec4p (Sec4) loaded with either GTPγS or GDP were incubated with a wild-type yeast cell extract (strain NY 1210). Copurifying proteins were subsequently eluted, subjected to SDS-PAGE electrophoresis, and silver stained. Only GST was used as a control (right). Proteins that exhibit preferential binding to GTPγS-Sec4p are indicated with arrows; arrowheads indicate proteins that preferentially bind to GDP-Sec4p. Asterisks indicate the GST-Sec4p and GST proteins.
Sro7p binds preferably to the activated, GTP-bound form of Sec4p
As shown in Table 1, Sro7p displayed fivefold higher mass spectrometry coverage when Sec4p was GTPγS bound compared with its GDP-bound form. To validate these findings, we repeated the affinity chromatography with an extract from yeast cells expressing an integrated HA3-tagged allele of Sro7p. The tag did not interfere with the functionality of the protein, as demonstrated by its ability to suppress the cold sensitivity of an sro7Δ sro77Δ double mutant and the salt sensitivity of an sro7Δ mutant strain (Fig. 2 A and not depicted). Western blot analysis confirmed that Sro7-HA3p migrates at its expected molecular weight (Fig. 2 B). An extract of this strain was incubated with GST-Sec4p in its different nucleotide-bound states or in the nucleotide-free state, GST-Ypt1p and GST. The latter two were used as specificity controls. Ypt1p is another member of the Rab GTPase family that is required for ER-to-Golgi transport in S. cerevisiae (Jedd et al., 1995). To test whether this method indeed distinguishes the different nucleotide-bound states and the nucleotide-free state of Sec4p, we used antibodies to probe for Sec2p and Sec15p. As mentioned above, Sec15p is the only previously documented effector of Sec4p, which was shown by two-hybrid analysis to bind preferentially to a hydrolysis-deficient (GTP locked) allele of Sec4p (Guo et al., 1999b). Sec2p is the exchange factor of Sec4p, which preferentially binds to the nucleotide-free state of Sec4p and, with lesser affinity, to the GDP-bound form of Sec4p (Walch-Solimena et al., 1997; Ortiz et al., 2002). As shown in Fig. 2 C, Sec15p binds specifically to the GTPγS-bound form of GST-Sec4p (α-Sec15), whereas Sec2p binds with the highest affinity to the nucleotide-free state of GST-Sec4p (α-Sec2), thus confirming the nucleotide specificity of the method. To detect Sro7-HA3p, these samples were analyzed by Western blotting with an α-HA antibody. Confirming the result obtained by mass spectrometry, Sro7-HA3p was found to bind much more efficiently to the GTPγS-bound form of GST-Sec4p than to its GDP-bound or nucleotide-free state (Fig. 2 C, α-HA).
Table I.
Mass spectrometry results for Sro7p
| Recovery of Sro7p | GTPγS-Sec4p | GDP-Sec4p |
|---|---|---|
| Percentage of protein sequence | 9.9 | 1.9 |
| Number of identified peptides | 7 | 1 |
| Identified peptides | R.TVFETEINVPQPDYIR.D | R.SSDDNNANHPEHQYTKPTRK.G |
| R.GDNQSLTMIDLGYTPR.Y | ||
| R.RGPAIIYMENIR.E | ||
| K.YPLAATGLSYISTVEK.N | ||
| K.YITESSVLR.N | ||
| R.VSEFQASLFSTVK.E | ||
| R.SSDDNNANHPEHQYTKPTRK.G |
Figure 2.
Sro7-HA 3 p from yeast extracts binds preferentially to GTPγS-Sec4p. (A) Expression of SRO7-HA 3 suppresses the cold sensitivity of an sro7Δ sro77Δ strain. Wild-type (WT), sro7Δ sro77Δ, and SRO7-HA 3 sro77Δ yeast strains were spotted in 10-fold dilutions onto YPD media plates and grown at the indicated temperatures. (B) Extracts of a wild-type or SRO7-HA 3 strain (HA) were subjected to Western blot analysis using an α-HA antibody. Ponceau staining is shown as a loading control. (C) Sro7-HA3p binds preferentially to GTPγS-Sec4p. GST-Sec4 (Sec4), GST-Ypt1 (Ypt1), or GST immobilized on glutathione beads were incubated with an extract of an SRO7-HA 3 strain, and copurifying proteins were subjected to Western blot analysis using the indicated antibodies. GST-Sec4p and GST-Ypt1p were either stripped of nucleotide (NF) or loaded with GTPγS (GTP) or GDP. Ponceau staining of the Western blot is shown as a loading control. The input lanes represent 0.2 and 0.3%, respectively.
Moreover, Sro7-HA3p does not bind to GST-Ypt1p or GST (Fig. 2 C, α-HA), establishing the specificity of the Sro7p–Sec4p interaction. Approximately 2–4% of total Sro7-HA3p was found to bind to GTPγS-Sec4p in this assay. The amount of Sro7p bound to GTP-Sec4p in this assay exceeds the amount of bound Sec15p (Fig. 2 C, compare α-Sec15 with α-HA). Interestingly, the same amount of Sro7-HA3p binds to GTPγS-Sec4p when only half the amount of extract is used (not depicted), suggesting that the amount of activated Sec4p available for Sro7p binding is limiting in this assay. Altogether, our data indicate that Sro7p from yeast extract binds preferentially to the GTP-bound form of Sec4p, confirming the result obtained by mass spectrometry.
Sro7p binds directly to Sec4p
We next determined whether purified Sro7p and Sec4p bind to each other in the absence of other proteins. Because Sro7p cannot be purified from bacteria (unpublished data), it was purified from yeast using a multistep procedure (see Purification of full-length Sec9 and Sro7 for details). This purification resulted in an Sro7p preparation that appears homogeneous by SDS-PAGE and Coomassie staining (Fig. 3 A) and dissociated from its binding protein, Sec9p (Lehman et al., 1999; unpublished data). As shown in Fig. 3 B, purified Sro7p binds to GST-Sec4p preferentially in the presence of GTPγS. The amount of Sro7p bound to GST-Sec4p ranges from ∼5 to 20% of total Sro7p bound to GTPγS-Sec4p, and 1/20–1/10 of this amount bound to the GDP-bound or nucleotide-free Sec4p (Fig. 3 B). The observed binding is specific because Sro7p does not bind to any of the nucleotide-bound and -free forms of GST-Ypt1 or GST alone (Fig. 3 B and not depicted). Thus, purified Sro7p binds to Sec4p in its activated state. Given the strength of the observed interaction (Fig. 3 B) and the purity of Sro7p (Fig. 3 A), we conclude that this interaction is very likely to be direct because any copurifying factor would be substoichiometric.
Figure 3.
Sro7p binds directly to Sec4p. (A) Sro7p was purified from yeast using a multistep purification protocol (see Materials and methods). 1 and 1.8 μg of purified Sro7p were subjected to SDS-PAGE analysis and stained with Coomassie. (B) 4 μM GTPγS-, GDP-bound, or nucleotide-free (NF) GST-Sec4 (Sec4) or GST alone in the presence of GTPγS on glutathione beads were incubated with 1 μM of purified Sro7p at 4°C. The beads were pelleted, and bound Sro7p was detected by Western blot analysis with an Sro7p-specific (α-Sro7) antibody. Coomassie staining of the GST fusion proteins as used in this assay is shown as a loading control. The input represents 10% of total Sro7p.
Sro7p and Sec4p interact in vivo
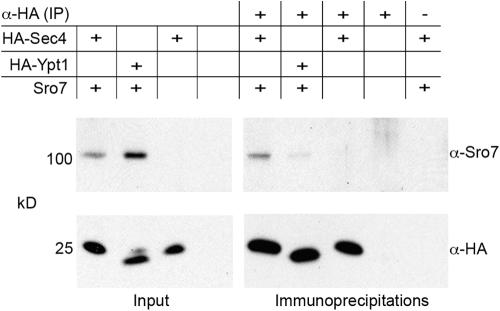
To test whether this interaction takes place in vivo, coimmunoprecipitation experiments were performed. Because of the low abundance of Sro7p (Ghaemmaghami et al., 2003; Huh et al., 2003), Sro7p and HA-tagged Sec4p or, as a control, HA-tagged Ypt1p, were cooverexpressed in yeast. The HA-tagged Rab proteins were immunoprecipitated using an α-HA antibody, and coimmunoprecipitating Sro7p was detected by Western blotting with an α-Sro7p antibody. As shown in Fig. 4, Sro7p coimmunoprecipitates with HA-Sec4p. This interaction is specific because (1) only a background amount of Sro7p is found to interact with Ypt1p; (2) the signal requires the cooverexpression of both Sec4p and Sro7p; and (3) no signal can be detected when the antibody is omitted from the precipitation reaction (Fig. 4). These data further support the conclusion that Sro7p binds directly to Sec4p because no third protein was overexpressed. Quantification of the interaction revealed that ∼2% of total Sro7p bound to GST-Sec4p (immunoprecipitated amount set to 100%). This relatively low amount might reflect the predominantly inactivated state of Sec4p in a yeast lysate. Together, our in vivo and in vitro data establish Sro7p as an effector of Sec4p.
Figure 4.
Sro7p coimmunoprecipitates with Sec4p. Yeast cells overexpressing HA-Sec4p and Sro7p, HA-Ypt1p and Sro7p, HA-Sec4p alone, or none of these proteins were lysed, and HA-Sec4p and HA-Ypt1p were immunoprecipitated using a rat α-HA antibody (α-HA [IP]). Proteins were subsequently subjected to Western blot analysis, and coimmunoprecipitating Sro7p was detected using an Sro7p-specific antibody (α-Sro7). A mouse α-HA antibody (α-HA) was used to detect the precipitated HA-Sec4p and HA-Ypt1p. The specificity of the observed coimmunoprecipitation was shown by omitting the antibody from the reaction (α-HA [IP], − lane). The amount of HA-Sec4p and HA-Ypt1p shown represents 5% of the immunoprecipitation. The inputs represent 0.15% of total protein.
Sec4p, Sro7p, and Sec9p form a trimeric complex
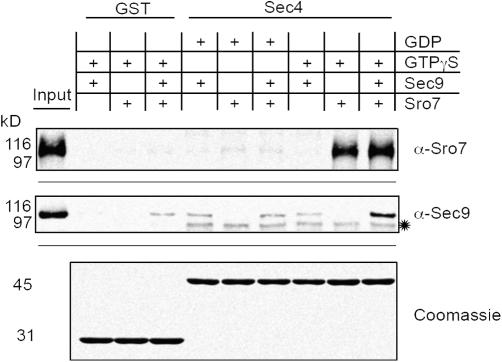
Sro7p and its paralogue, Sro77p, belong to the lgl tumor suppressor family (Kagami et al., 1998; Larsson et al., 1998; Lehman et al., 1999). Both proteins have been found to localize to the plasma membrane and to be required for exocytosis in yeast (Kagami et al., 1998; Larsson et al., 1998; Lehman et al., 1999). Moreover, it has been shown that Sro7p binds to the plasma membrane t-SNARE Sec9p and that this interaction is required for Sro7p's function (Lehman et al., 1999; Gangar et al., 2005). Although no direct, GTP-specific interaction of Sec4p with SNAREs was previously found (Grote and Novick, 1999), our data suggested the possibility that GTP-Sec4p indirectly signals to SNAREs via Sro7p. To explore this possibility, we performed in vitro binding assays using recombinant GST-Sec4p, Sec9p, and purified Sro7p. As shown in Fig. 5, Sec9p interacts with Sec4p, but only in the presence of GTPγS and Sro7p. Under the conditions used in this assay, ∼5–10% of the input Sro7p binds to Sec4p. Only background binding of Sec9p to GTPγS-Sec4p was observed when Sro7p was omitted from the binding assay, suggesting that Sec9p binds to Sec4p through Sro7p and implying that Sec4p and Sec9p use different binding sites on Sro7p (Fig. 5). The specificity of this interaction was demonstrated by the fact that no significant binding of Sro7p or Sec9p to GDP-Sec4p or GST was detected (Fig. 5, GST). Therefore, our data imply that GTP-Sec4p, Sro7p, and Sec9p are able to form a ternary complex.
Figure 5.
Sec4p, Sro7p, and Sec9p form a nucleotide-dependent ternary complex. Glutathione beads with 4 μM of either GTPγS- or GDP-loaded GST-Sec4p (Sec4) or GST alone in the presence of GTPγS were incubated with either purified Sro7p (Sro7) or recombinant Sec9-His6p (Sec9) or both at 1 μM concentrations at 4°C. Copurifying Sro7p or Sec9-His6p were detected by Western blotting using an Sro7p-specific (α-Sro7) or Sec9p-specific (α-Sec9) antibody. The input lanes represent 5% of the total Sro7p or Sec9-His6p. Coomassie staining is shown as a loading control. The asterisk indicates a contaminating protein present on the GST-Sec4 beads that cross reacts with the α-Sec9 antibody.
Sro7p interacts genetically with Sec3p, a subunit of the exocyst complex
Our data suggest that Sec4p might play a role in SNARE regulation via its effector, Sro7p. The other known effector of Sec4p is Sec15p, a member of the exocyst complex (Guo et al., 1999b). The exocyst is required for the tethering of secretory vesicles to the plasma membrane (TerBush et al., 1996; Guo et al., 1999a), a step that precedes the final SNARE-mediated fusion of those vesicles with the plasma membrane. If Sro7p and the exocyst act in converging pathways, each downstream of Sec4p, the overexpression of Sro7p might be expected to compensate for the loss of exocyst function, and, conversely, the loss of Sro7p would exacerbate the phenotype of exocyst mutants. As shown in Fig. 6 A, overexpression of SRO7 on a 2μ plasmid partially rescues the temperature sensitivity of a sec3Δ mutant. In agreement with this, it has recently been published that the overexpression of SRO7 rescues the exocytosis defect of the sec3Δ mutant (Zhang et al., 2005). Interestingly, however, the level of suppression achieved by overexpression of SRO7 is not as strong as that achieved by 2μ SEC4 (Fig. 6 A, compare sec3Δ 2μ SRO7 with sec3Δ 2μ SEC4). We found that the deletion of SEC3 increases the doubling time of a yeast strain about twofold compared with wild-type yeast at 30°C in synthetic complete (SC) minimal medium (225 ± 21 min vs. 125 ± 7 min; Fig. 6 B). Overexpression of SEC4 rescues this growth defect to nearly wild-type levels (145 ± 7 min), but sec3Δ cells overexpressing SRO7 still display a strong growth defect (190 ± 14 min; Fig. 6 B). Similarly, 2μ SRO7 does not suppress the lethality of sec3Δ on yeast peptone dextrose (YPD) media (Fig. 6 C; Wiederkehr et al., 2003). One interpretation of these data is that Sec4p signals to other effectors in addition to Sro7p and the exocyst to achieve vesicle fusion. In support of our suggestion of converging functions for Sro7p and the exocyst, deletion of SRO7 in a sec3Δ strain further aggravates its growth defect (Fig. 7 A). We observed that the doubling time of a sec3Δ sro7Δ strain (360 ± 28 min) is almost twice that of a sec3Δ single mutant strain (225 ± 21 min; Fig. 7 B). This growth phenotype is accompanied by a significant drop in exocytosis in the sec3Δ sro7Δ mutant of ∼10% compared with the sec3Δ mutant (73 ± 7% vs. 60 ± 5% of secreted invertase; Fig. 7 C). This further implicates Sro7p and the exocyst in interrelated functions on the exocytic pathway.
Figure 6.
Overexpression of SRO7 partially suppresses the growth defect of a sec3Δ strain. (A) Wild-type (WT), sec3Δ, and sec3Δ yeast strains overexpressing either SEC4 (sec3Δ 2μ SEC4) or SRO7 (sec3Δ 2μ SRO7) from a 2μ plasmid were spotted onto SC media plates in 10-fold dilutions and grown at the indicated temperatures. (B) Wild-type, sec3Δ, and sec3Δ yeast strains overexpressing either SEC4 (sec3Δ 2μ SEC4) or SRO7 (sec3Δ 2μ SRO7) on 2μ plasmids were grown at 30°C in SC media. The doubling time was measured by plotting OD600 over time. The mean of three independent experiments is shown. Error bars represent SEM. (C) Wild-type, sec3Δ, and sec3Δ yeast strains overexpressing either SEC4 (sec3Δ 2μ SEC4) or SRO7 (sec3Δ 2μ SRO7) on a 2μ plasmid were spotted onto a YPD plate in 10-fold dilutions and grown at 24°C.
Figure 7.
Loss of SRO7 exacerbates the sec3Δ phenotype. (A) Wild-type (WT), sro7Δ, sec3Δ, and sec3Δ sro7Δ yeast strains were spotted onto SC media plates in 10-fold dilutions and grown at the indicated temperatures. (B and C) Wild-type, sec3Δ, and sec3Δ sro7Δ yeast strains were grown at 30°C in SC media. (B) The doubling time was measured by plotting OD600 over time. The mean of three independent experiments is shown. (C) Deletion of SRO7 in a sec3Δ strain leads to a more pronounced defect in exocytosis. Expression of the enzyme invertase was derepressed by shifting the strains into low glucose medium, and secretion of invertase was measured after 1 h of derepression by a colorimetric test (see Materials and methods). Values indicate percentages of secreted invertase. A mean of four different experiments is shown. Error bars represent SEM.
Because we observed a synthetic growth defect in the sec3Δ sro7Δ mutant strain, we decided to test whether the deletion of SRO7 would have a similar influence on the growth of other exocyst mutant strains. For that purpose, we used a collection of temperature-sensitive late secretory mutant strains (sec1-1, sec2-41, sec3-2, sec4-8, sec5-24, sec6-4, sec8-9, sec9-4, sec10-2, and sec15-1). We observed that sec2-41, sec3-2, sec4-8, sec8-9, sec9-4, and sec15-1 in combination with sro7Δ displayed slower growth compared with the single mutant strains at the permissive temperature of 24°C (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200510016/DC1). Interestingly, the most striking feature we observed is that combining a deletion of SRO7 with almost all of the heat-sensitive mutants lead to a synthetic growth defect at 14°C (Table II and Fig. S1). Double deletion of SRO7 and its paralogue SRO77 has been found to lead to cold sensitivity of the resulting double mutant strain (Kagami et al., 1998; Lehman et al., 1999), which demonstrated that their gene products share a common function. We found that the double mutants of the Rab GTPase Sec4p (sec4-8) or its GEF Sec2p (sec2-41) with sro7Δ display synthetic lethality at 14°C (Table II and Fig. S1). This result further indicates a common function of Sro7p and Sec4p. The fact that sro7Δ causes sec1-1, a heat-sensitive mutant of the SNARE-interacting protein Sec1p, and sec9-4, a mutant of the t-SNARE Sec9p, to become cold sensitive (Table II and Fig. S1) adds genetic evidence for a role of Sro7p in SNARE function in yeast. Interestingly, we also found that the deletion of SRO7 leads to cold sensitivity in only a subset of the temperature-sensitive exocyst mutant strains (Table II and Fig. S1). These data might either reflect the relative strength of these mutant alleles at low temperature or indicate that only those subunits share a function with Sro7p and, therefore, add further evidence for a functional specialization of exocyst subunits within the complex (Wiederkehr et al., 2004). Altogether, our genetic data support a possible role for Sro7p in transferring the signal of the Rab GTPase Sec4p to SNARE function in yeast.
Table II.
Synthetic growth defects of temperature-sensitive secretory mutant strains when combined with sro7 Δ
| Mutant | Growth at 14°C |
|---|---|
| sec1-1 sro7Δ | Slow |
| sec9-4 sro7Δ | Slow |
| sec2-41 sro7Δ | None |
| sec4-8 sro7Δ | None |
| sec3-2 sro7Δ | None |
| sec5-24 sro7Δ | Wild type–like |
| sec6-4 sro7Δ | Wild type–like |
| sec8-9 sro7Δ | None |
| sec10-2 sro7Δ | Slowa |
| sec15-1 sro7Δ | None |
Single temperature-sensitive mutant strains grow comparably with wild-type yeast at 14°C. Double temperature-sensitive sro7Δ mutant strains display the indicated synthetic growth phenotypes at this temperature after growth for 7 d.
Only slight growth defects were observed.
Genetic evidence for a role of Sro7p downstream of Sec4p
Although Sec3p is the only nonessential exocyst protein (Wiederkehr et al., 2003), a previous study revealed that SEC5 and EXO70 also could be deleted if either Sec4p or Sec1p were overproduced (Wiederkehr et al., 2004). Given our data that overproduction of Sro7p rescues the growth defect of a sec3Δ mutant strain, we hypothesized that 2μ SRO7 might also be able to overcome the lethality of exo70Δ and sec5Δ mutant strains. As shown in Fig. 8, overexpression of SRO7 on a 2μ plasmid indeed rescues the lethality of both exo70Δ (Fig. 8 A) and sec5Δ (Fig. 8 B) mutant strains. Because we found that Sro7p is an effector of Sec4p with an exocyst-related function, it appeared likely that Sro7p would be required for the Sec4p-mediated rescue of exo70Δ and sec5Δ mutant strains (Wiederkehr et al., 2004). Indeed, the deletion of SRO7 reduces the growth of both exo70Δ and sec5Δ strains rescued by the overexpression of SEC4 from a 2μ plasmid (Fig. 9, A and B; compare exo70Δ 2μ SEC4 or sec5Δ 2μ SEC4 with exo70Δ sro7Δ 2μ SEC4 or sec5Δ sro7Δ 2μ SEC4). We found that the doubling times of the exo70Δ sro7Δ 2μ SEC4 (240 ± 21 min) or sec5Δ sro7Δ 2μ SEC4 (242 ± 31 min) yeast strains are significantly increased compared with the yeast strains without additional deletion of SRO7 (190 ± 14 min and 185 ± 21 min, respectively; Fig. 9, C and D). In striking contrast, deletion of SRO7 was found to only have minor influences on the growth of both exo70Δ and sec5Δ mutant strains rescued by overexpression of SEC1 from a 2μ plasmid on either solid or liquid media (Fig. 9, compare exo70Δ 2μ SEC1 or sec5Δ 2μ SEC1 with exo70Δ sro7Δ 2μ SEC1 or sec5Δ sro7Δ 2μ SEC1). Therefore, these data provide genetic evidence that Sro7p functions downstream of Sec4p in an exocyst-related function. They further imply that although Sec4p signaling is upstream of Sro7p and the exocyst, Sec1p functions downstream of both (summarized in the model in Fig. 10). Our results are also consistent with previous data that showed that Sec4p and Sec1p use different mechanisms for suppression of exocyst deletion mutations (Wiederkehr et al., 2004).
Figure 8.
Overexpression of SRO7 suppresses the lethality of deletion of EXO70 and SEC5. (A) Wild-type (WT) and exo70Δ yeast strains overexpressing either SEC4 (exo70Δ 2μ SEC4), SEC1 (exo70Δ 2μ SEC1), or SRO7 (exo70Δ 2μ SRO7) were spotted onto SC media plates in 10-fold dilutions and grown at the indicated temperatures. (B) Wild-type and sec5Δ yeast strains overexpressing either SEC4 (sec5Δ 2μ SEC4), SEC1 (sec5Δ 2μ SEC1), or SRO7 (sec5Δ 2μ SRO7) were spotted onto SC media plates in 10-fold dilutions and grown at the indicated temperatures.
Figure 9.
Sro7p is required for the suppression of exo70 Δ and sec5Δ lethality by the overexpression of SEC4. (A and B) Wild-type (WT) yeast, exo70Δ (A) or sec5Δ (B) single, and exo70Δ sro7Δ (A) or sec5Δ sro7Δ (B) double mutant strains overexpressing SEC4 (2μ SEC4) or SEC1 (2μ SEC1) were spotted onto SC media plates in 10-fold dilutions and grown at the indicated temperatures. (C and D) Wild-type yeast, exo70Δ (C) or sec5Δ (D) single, and exo70Δ sro7Δ (C) or sec5Δ sro7Δ (D) double mutant strains overexpressing SEC4 (2μ SEC4) or SEC1 (2μ SEC1) were grown at 30°C in SC media. The doubling time was measured by plotting OD600 over time. The mean of two independent experiments is shown. Error bars represent SEM.
Figure 10.
Model for the Sec4p signaling pathways. Secretory vesicles (V) carry the Rab GTPase Sec4p and its GEF Sec2p, which keeps Sec4p in its activated, GTP-bound state. Sec15p, a member of the exocyst complex, is one effector for Sec4p, and the interaction of these two proteins is required for the assembly of this complex and its tethering function in exocytosis (Guo et al., 1999b). Sec1p interacts with the exocyst (Wiederkehr et al., 2004) and binds to assembled SNARE complexes, possibly stabilizing them (Carr et al., 1999). Another effector of Sec4p, Sro7p (this study), interacts with the exocytic t-SNARE Sec9p (Lehman et al., 1999), and genetic data indicate that this interaction is required for Sec4p's role in exocytosis (Brennwald et al., 1994; Lehman et al., 1999; and this study). A recent study showed that the exocyst and the yeast lgl family members interact (Zhang et al., 2005), allowing an integrated response. Arrows indicate physical interactions.
Sro7p does not bypass all exocyst functions
Given the fact that both Sro7p and the exocyst are Sec4p effectors and that the overexpression of SRO7 suppresses the phenotypes of three different exocyst deletions, it appeared possible that Sro7p would be able to completely bypass the exocyst, which would imply that Sro7p and the exocyst have identical functions. We assessed this possibility by investigating whether the overexpression of SRO7 (or, if necessary, SRO7 in combination with SEC4) would rescue the lethality of a sec15Δ strain. Sec15p is the subunit of the exocyst that directly interacts with Sec4p (Guo et al., 1999b). It has been previously shown that the overexpression of SEC4 and SRO7 was able to suppress the temperature sensitivity of a sec15-1 mutant strain (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200510016/DC1; Salminen and Novick, 1987; Guo et al., 1999b; Lehman et al., 1999). However, under all tested conditions (different media and temperatures), neither 2μ SRO7 nor 2μ SRO7 in combination with 2μ SEC4 were able to suppress the lethality of a sec15Δ strain (Fig. S2 B and not depicted). These genetic data indicate that Sro7p shares some, but not all, functions with the exocyst in yeast.
Discussion
Sro7p is an effector of the secretory Rab GTPase Sec4p
Sec4p is a member of the Rab GTPase family that plays important roles in the yeast secretory pathway (Salminen and Novick, 1987; Goud et al., 1988). Only one effector for Sec4p was known before this study: the exocyst subunit Sec15p (Guo et al., 1999b). We described the identification by affinity chromatography of a second Sec4p effector, Sro7p, which is a member of the lgl family of tumor suppressors (Kagami et al., 1998; Larsson et al., 1998; Lehman et al., 1999). We have shown that Sec4p and Sro7p interact in vitro and in vivo and that the interaction requires Sec4p to be in its activated, GTP-bound state (Figs. 2–5). Our data establish Sro7p as an effector of Sec4p and provide further support for a role of lgl family members in membrane traffic (see Introduction).
Several previously published reports support our findings. Sro7p and its paralogue Sro77p were originally identified as high-copy suppressors of Rho3p (Matsui and Toh-e, 1992a; Kagami et al., 1998), which is a Rho GTPase required for actin cytoskeleton polarity and polarized exocytosis in yeast (Matsui and Toh-e, 1992b; Imai et al., 1996; Adamo et al., 1999). Another protein found in this screen (Sro6p) was subsequently identified as Sec4p. These genetic data suggest that the identified proteins positively influence cell polarity and/or polarized exocytosis, as had been shown for Sec4p (Salminen and Novick, 1987; Goud et al., 1988). Subsequently, Sro7/77p have also been found to be required for exocytosis in yeast (Lehman et al., 1999). Additionally, the overexpression of Sro7p was found to suppress the cold sensitivity of a sec4-P48 mutant (Brennwald et al., 1994), which suggested that Sro7p might act downstream of Sec4p function.
Sro7p genetically interacts with the exocyst downstream of Sec4p
Genetic data presented in this study indicate that the function of Sro7p partially overlaps with that of the exocyst (Figs. 6–9), an eight-subunit vesicle-tethering complex required for tethering secretory vesicles to the plasma membrane (TerBush et al., 1996; Guo et al., 1999a). We found that the overexpression of SRO7 suppresses the growth defects of three different exocyst deletion mutants (sec3Δ, sec5Δ, and exo70Δ; Figs. 6 and 8), extending recently published data (Zhang et al., 2005). We also showed that the deletion of SRO7 impairs the growth and secretory function of a sec3Δ strain (Fig. 7), which further implies that Sro7p and the exocyst function in concert.
We recently established that the overexpression of either Sec4p or Sec1p can bypass the inviability of sec5Δ or exo70Δ strains (Wiederkehr et al., 2004). Interestingly, we now demonstrate that the deletion of SRO7 in exo70Δ or sec5Δ strains reduces growth only when SEC4 is overexpressed (Fig. 9). If SEC1 is overexpressed, no significant difference in growth can be detected upon SRO7 deletion (Fig. 9). These data provide genetic evidence that Sro7p acts downstream of Sec4p in its capacity as a suppressor and demonstrate that Sro7p is involved in a Sec4p- and exocyst-related function (Fig. 10). Moreover, they further strengthen the findings by Wiederkehr et al. (2004) that indicate that Sec4p and Sec1p use different mechanisms in their suppression of the two exocyst mutants.
Although SRO7, SEC1, and SEC4 can each act as a high-copy suppressor of sec3Δ, sec5Δ, and exo70Δ, there is no simple hierarchy in the efficiency of suppression. Thus, SEC4 suppresses sec3Δ and sec5Δ better than does SRO7 (Figs. 6 and 8), but SRO7 is somewhat better at suppressing exo70Δ than is SEC4 (Fig. 8). Furthermore, SEC1 suppresses exo70Δ much better than does SEC4, but SEC4 suppresses sec5Δ much better than does SEC1 (Fig. 8). Although this pattern is presently difficult to interpret, it does support the notion that different subunits of the exocyst fulfill distinct functions (Wiederkehr et al., 2004), possibly in tethering and regulation of SNARE function.
As mentioned above, the Sec15p subunit of the yeast exocyst is also a Sec4p effector (Guo et al., 1999b). If Sro7p and the exocyst have purely redundant functions downstream of Sec4p, the overexpression of one effector should overcome the loss of the other. Contrary to this prediction, we found that overexpression of SRO7, either alone or in combination with SEC4, did not suppress the lethality of a sec15Δ mutant under all conditions tested (Fig. S2 B and not depicted). Thus, these data indicate that Sro7p and the exocyst have interrelated but not identical functions. In agreement with this, the overexpression of individual exocyst subunits did not suppress the salt sensitivity of the sro7Δ mutant (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200510016/DC1) or the cold sensitivity of an sro7Δ sro77Δ mutant (Zhang et al., 2005). Although it might simply be that more than one subunit (i.e., a subcomplex) is required for suppression, another explanation for this finding is that Sro7p and Sro77p have important cellular functions beyond their role in exocytosis. In agreement with this, overexpression of either of the t-SNAREs, SEC9 and SSO1, or the SNARE regulator SEC1 failed to suppress the sro7Δ sro77Δ phenotype (Zhang et al., 2005). Sro7/77p have been shown to interact biochemically with the yeast type II myosin Myo1p, an interaction that appears to play a role in the remodeling of the actin cytoskeleton (Kagami et al., 1998), which is consistent with data concerning lgl family members from other organisms (see Introduction). Another explanation for the failure of overexpression of single exocyst subunits to suppress the salt or cold sensitivity of Sro7/77p mutants could be that Sro7p and Sro77p act downstream of some, but not all, aspects of exocyst function (Wiederkehr et al., 2004; Zhang et al., 2005).
Sro7p links a Rab (Sec4p) to a t-SNARE (Sec9p)
Rab GTPase activity has previously been implicated in the regulation of SNARE complex assembly (Lian et al., 1994; Sogaard et al., 1994). Furthermore, genetic evidence suggested that plasma membrane SNAREs act in response to Rabs because the overexpression of SEC9 has been found to suppress the growth defect of a sec4-P48 mutant strain (Brennwald et al., 1994). Nonetheless, a direct interaction of activated Sec4p and the exocytic SNAREs could not be detected (Grote and Novick, 1999). We provide evidence in this study that GTP-bound Sec4p interacts with the plasma membrane SNAREs via Sro7p. The Sec4p effector Sro7p was found to interact with the t-SNARE Sec9p (Lehman et al., 1999), and recent data indicate that this interaction is important for Sro7p's function in secretion (Gangar et al., 2005). We have demonstrated that Sec9p can associate with Sec4p, but only in the presence of GTPγS and Sro7p (Fig. 5). This interaction provides a link between Rab signaling and SNARE function in yeast exocytosis.
Together, two convergent signaling pathways from the Rab GTPase Sec4p appear to lead to vesicle fusion in yeast (Fig. 10). Secretory vesicles carrying activated Sec4p transmit a signal to Sec15p, which leads to exocyst assembly and vesicle tethering (Guo et al., 1999b). A recently documented association of Sec1p with the exocyst (Wiederkehr et al., 2004) potentially links this pathway to SNARE function. In a second branch of the pathway, Sec4p transmits a signal through the lgl family member Sro7p. Because Sec4p, Sro7p, and the t-SNARE Sec9p can assemble into a ternary complex, we propose that Sro7p conveys the signal from the Rab GTPase to SNARE function in yeast. These interactions may normally occur after vesicle tethering by the exocyst. There appears to be crosstalk between these two pathways because Sro7p has recently been shown to associate with the exocyst subunit Exo84p (Zhang et al., 2005). Further studies will be necessary to explore these proposals.
Materials and methods
Yeast strains and genetic analysis
S. cerevisiae strains used in this study are listed in Table III. Standard techniques and media were used for growth, mating, sporulation, tetrad dissection, and yeast transformation (Ito et al., 1983; Sherman, 1991). Most yeast strains were created by mating appropriate parent strains. The resulting diploids were sporulated and dissected, and appropriate spores were picked. Strain NY 2587 was created by using a PCR-based method (Longtine et al.,1998). NY 2592–2595 were made by transforming combinations of plasmids pNB 529, pNB 833, or pNB 829 and either pNB 530 or pNB 1246 into NY 1210. Overexpression of HA-Sec4p, HA-Ypt1p, and Sro7p was confirmed by Western blotting. NY 2602 and 2603 were created by transforming pNB 142 or pB 745 into the appropriate diploid yeast strain before dissection. Strains NY 2609 and NY 2610 were made by streaking diploids (NY 2590 × NY 2478 or NY 2590 × NY 2476) onto SC medium containing 5-FOA (1 mg/ml final concentration) to select cells that lost the 2μ SEC1 plasmid. Subsequently, these diploid cells were transformed with plasmid pB 745, were sporulated and dissected, and the appropriate spores were picked.
Table III.
Yeast strains
| Yeast strain | Description | Source |
|---|---|---|
| NY 1210 | Mata ura3-52 leu2-3,112 his3-Δ200 GAL+ | P. Novick collection |
| BY 570 | Matα sro7Δ::LEU2 leu2-3,112 ura3-52 his3-Δ200 | Lehman et al., 1999 |
| BY 569 | Mata sro77Δ::URA3 leu2-3,112 ura3-52 his3-Δ200 | Lehman et al., 1999 |
| NY 2587 | Mata SRO7-HA 3::kanMX ura3-52 leu2-3,112 his3-Δ200 GAL+ | This study |
| NY 2588 | Mata sro7Δ::LEU2 sro77Δ::URA3 leu2-3,112 ura3-52 his3-Δ200 | This study; based on Lehman et al., 1999 |
| NY 2589 | Mata SRO7-HA 3::LEU2 sro77Δ::URA3 leu2-3,112 ura3-52 his3-Δ200 | This study |
| NY 2592 | Mata P Gal1::SRO7::URA3 PGal1::HASEC4::LEU2 ura3-52 leu2-3,112 his3-Δ200 GAL+ | This study |
| NY 2593 | Mata P Gal1::URA3 PGal1::HA-SEC4::LEU2 ura3-52 leu2-3,112 his3-Δ200 GAL+ | This study |
| NY 2594 | Mata P Gal1::SRO7::URA3 PGal1::HA-YPT1::LEU2 ura3-52 leu2-3,112 his3-Δ200 GAL+ | This study |
| NY 2595 | Mata P Gal1::URA3 PGal1::LEU2 ura3-52 leu2-3,112 his3-Δ200 GAL+ | This study |
| NY 2448 | Matα sec3Δ::kanMX ura3-52 leu2-3,112 his3-Δ200 | Wiederkehr et al., 2003 |
| NY 2601 | Mata sec3Δ::kanMX sro7Δ::LEU2 ura3-52 leu2-3,112 his3-Δ200 | This study |
| NY 2602 | Matα sec3Δ::kanMX ura3-52 leu2-3,112 his3-Δ200 2μ SEC4::URA3 | This study |
| NY 2603 | Matα sec3Δ::kanMX ura3-52 leu2-3,112 his3-Δ200 2μ SRO7::URA3 | This study |
| NY 2479 | Matα exo70Δ::kanMX leu2-3,112 ura3-52 2μ SEC4::URA3 | Wiederkehr et al., 2004 |
| NY2478 | Matα exo70Δ::kanMX leu2-3,112 ura3-52 2μ SEC1::URA3 | Wiederkehr et al., 2004 |
| NY 2604 | Matα exo70Δ::kanMX sro7Δ::LEU2 leu2-3,112 ura3-52 2μ SEC4::URA3 | This study |
| NY 2605 | Matα exo70Δ::kanMX sro7Δ::LEU2 leu2-3,112 ura3-52 2μ SEC1::URA3 | This study |
| NY 2477 | Matα sec5Δ::kanMX leu2-3,112 ura3-52 2μ SEC4::URA3 | Wiederkehr et al., 2004 |
| NY 2476 | Matα sec5Δ::kanMX leu2-3,112 ura3-52 2μ SEC1::URA3 | Wiederkehr et al., 2004 |
| NY 2607 | Mata sec5Δ::kanMX sro7Δ::LEU2 leu2-3,112 ura3-52 2μ SEC4::URA3 | This study |
| NY 2608 | Matα sec5Δ::kanMX sro7Δ::LEU2 leu2-3,112 ura3-52 2μ SEC1::URA3 | This study |
| NY 2609 | Matα exo70Δ::kanMX leu2-3,112 ura3-52 2μ SRO7::URA3 | This study |
| NY 2610 | Matα sec5Δ::kanMX leu2-3,112 ura3-52 2μ SRO7::URA3 | This study |
| NY 68 | Mata sec15-1 his4-619 ura3-52 | P. Novick collection |
| NY 2421 | sec15Δ::kanMX/SEC15 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/MET15 lys2Δ0/LYS2 | EUROSCARF collection |
| NY 605 | Mata ura3-52 leu2-3,112 Gal+ | P. Novick collection |
| NY 768 | Matα sec1-1 ura3-52 leu2-3,112 | P. Novick collection |
| NY 770 | Matα sec2-41 ura3-52 leu2-3,112 | P. Novick collection |
| NY772 | Mata sec3-2 ura3-52 leu2-3,112 | P. Novick collection |
| NY 774 | Matα sec4-8 ura3-52 leu2-3,112 | P. Novick collection |
| NY 776 | Matα sec5-24 ura3-52 leu2-3,112 | P. Novick collection |
| NY 778 | Matα sec6-4 ura3-52 leu2-3,112 | P. Novick collection |
| NY 780 | Matα sec8-9 ura3-52 leu2-3,112 | P. Novick collection |
| NY 782 | Mata sec9-4 ura3-52 leu2-3,112 | P. Novick collection |
| NY 784 | Mata sec10-2 ura3-52 leu2-3,112 | P. Novick collection |
| NY 786 | Mata sec15-1 ura3-52 leu2-3,112 | P. Novick collection |
| NY 2611 | Mata sec1-1 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2612 | Mata sec2-41 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2613 | Matα sec3-2 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2614 | Matα sec4-8 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2615 | Matα sec5-24 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2616 | Matα sec6-4 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2617 | Matα sec8-9 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2618 | Mata sec9-4 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2619 | Mata sec10-2 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
| NY 2620 | Mata sec15-1 sro7Δ::LEU2 ura3-52 leu2-3,112 | This study |
For genetic analysis, at least two different spores were analyzed per experiment. For dot spot analysis, stationary yeast cultures were diluted to an OD600 of ∼0.3, and 10-fold dilutions were spotted onto YPD media or SC plates and incubated at the indicated temperatures for 2–3 (25°C and above) or 7–8 (14°C) d.
Plasmids
Standard techniques were used for plasmid construction. The SEC4 ORF was amplified by PCR from genomic DNA and subsequently inserted into plasmid pGEX5X-1 (GE Healthcare) to create the GST-SEC4 expression vector (pNB 1245). The plasmid carrying GST-YPT1 was described previously (Wang et al., 2000).
The plasmid carrying SRO7 behind the GAL1 promoter (pNB 1246) was created by inserting the SRO7 ORF into plasmid pNB 530 (GAL1 promoter, ADH1 terminator, and URA3 marker). The plasmids carrying HA-SEC4 (pNB 833) and HA-YPT1 (pNB 829) behind the GAL1 promoter and the control plasmid pNB 529 have been described previously (Grote and Novick, 1999).
Plasmid pB 745 (2μ SRO7::URA3) was described previously (Lehman et al., 1999). 2μ plasmids pNB 142, 680, 807, 690, 216, 328, 685, 148, 887, and 888 were used to overexpress SEC4, SEC1, SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70, or EXO84, respectively (P. Novick collection).
Antibodies
The α-Sro7p antibody was described previously (Lehman et al., 1999). Clones 3F10 (rat monoclonal; Roche) and 16B12 (mouse monoclonal; Convance) were used as antibodies against the HA epitope. Rabbit polyclonal antibodies were used for the detection of Sec2p or Sec15p (P. Novick collection). A goat α-GST antibody (Sigma-Aldrich) was used to detect GST fusion proteins.
Yeast extract for Sec4p affinity chromatography
27 liters of wild-type yeast culture were lysed by homogenization in a microfluidizer (Microfluidics Corporation) in 140 ml of nucleotide-binding (NB) buffer (20 mM Hepes, pH 7.2, 100 mM KCl, 5 mM MgCl2, and 1 mM DTT) containing 1 mM PMSF, 5 μg/ml pepstatin A, and complete protease inhibitor EDTA-free cocktail (Roche). Triton X-100 was added to 1%, and unbroken cells and debris were eliminated by centrifugation at 10,000 g for 25 min at 4°C. The extract was dialyzed against NB buffer overnight. The concentration of the extract was adjusted to 40 mg/ml.
Purification of GST-Sec4, GST-Ypt1, and GST and nucleotide exchange
GST fusion proteins were purified from BL21 Escherichia coli strains (Novagen) as described previously (Christoforidis and Zerial, 2000; Wang et al., 2000; Idrissi et al., 2002). For nucleotide loading, GST-Sec4p or GST-Ypt1p were incubated with a 200-fold excess of either GTPγS or GDP in NB for 2 h at 30–37°C. To obtain the nucleotide-free state, the proteins were incubated in NB buffer supplemented with 10 mM EDTA.
Purification of full-length Sec9 and Sro7
Full-length Sec9p tagged with COOH-terminal His6 tag was purified from E. coli as described previously (Gangar et al., 2005). Sro7p with an NH2-terminal protein A/tobacco etch virus (TEV) tag was isolated from lysates prepared from overexpressing yeast strains using affinity chromatography with IgG Sepharose beads. It was then eluted by cleavage of the protein A tag with TEV protease and subsequently purified by ion exchange chromatography to apparent homogeneity based on SDS-PAGE analysis.
Sec4p affinity chromatography
The protocol was adapted from Christoforidis and Zerial (2000). In brief, 100 μl GTPγS- or GDP-bound GST-Sec4p or GST-containing beads (∼750 μg of protein) were incubated with 25 ml of wild-type yeast extract (40 mg/ml) for 2 h at 4°C. After several washings, bound proteins were eluted with elution buffer (20 mM Hepes, pH 7.2, 1.5 M NaCl, 20 mM EDTA, and 1 mM DTT) supplemented with 5 mM of the opposing nucleotide. The proteins were TCA precipitated, dried, and analyzed by mass spectrometry.
For the experiment in Fig. 2, 2.5 μl of loaded beads (∼15 μg) were incubated with 1 ml of a 20-mg/ml yeast extract (lysed using a French press; Sim-Amico Spectronic Instruments). Bound proteins were analyzed by Western blotting.
Mass spectrometry
Samples were suspended in 8 M urea and 100 mM Tris, pH 8.5, reduced with 100 mM TCEP, and cysteines were alkylated with 55 mM iodoacetamide. Lys-C was used to digest the proteins for 4 h at 37°C at a concentration of 1 μg/100 μl. CaCl2 was added to ensure tryptic specificity at 1 mM, and trypsin was used to digest the samples further at 1 μg/100 μl. The digests were then analyzed by μLC/μLC-MS/MS using an ion trap mass spectrometer (LCQ Deca; ThermoElectron). Multidimensional chromatography was performed online according to MacCoss et al. (2002) using the following salt steps of 500 mM ammonium acetate: 10, 25, 35, 50, 65, 80, and 100%. Tandem mass spectra were collected in a data-dependent fashion by collecting one full MS scan (m/z range = 400–1,600) followed by MS/MS spectra of the three most abundant precursor ions. The collection of resulting spectra was then searched against a database of yeast ORFs obtained from the Saccharomyces genome database (release date 08/27/04) using the SEQUEST algorithm (Eng et al., 1994). Peptide identifications were organized and filtered using the DTASelect program (Tabb et al., 2002). Filtering criteria for positive protein identifications in the Smt3p purification were the identification of two unique, fully tryptic peptides with Xcorr values >2.0 for +1 spectra, 2.2 for +2 spectra, and 3.75 for +3 spectra.
In vitro binding assays
Glutathione beads carrying 6 μM GST-Sec4p or GST were washed with 20 mM Tris, pH 7.5, 100 mM NaCl, and 1 mM DTT and were incubated in 20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, and 1 mM DTT in the presence of either 100 μM GTPγS, 100 μM GDP, or no nucleotide for 15 min at 25°C. Then, MgCl2 was added to a final concentration of 25 mM and incubated 45 min at 25°C. Binding assays were performed in binding buffer (20 mM Tris-HCl, pH 7.4, 140 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 0.5% Triton X-100). The final concentrations of GST/GST-Sec4p in the binding reactions were 4 μM, whereas concentrations of Sro7p or Sec9p were 1 μM. Samples were incubated at 4°C for 1 h. The beads were washed four times with binding buffer, and bound proteins were subjected to Western blot analysis. Sro7p and Sec9p were detected with rabbit α-Sro7 or α-Sec9 antibodies and α-rabbit IgG conjugated to AlexaFluor680 and were analyzed on an Odyssey Infrared Imaging System (LI-COR). For the experiment in Fig. 5, Sro7p–Sec9p mixtures (or Sro7p or Sec9p alone) were preincubated on ice for 1 h to allow the formation of binary complexes before their addition to the coated glutathione beads.
Immunoprecipitation
Yeast strains 2,592–2,595 were grown overnight in yeast peptone media containing raffinose at 25°C to an OD600 of ∼0.4. Production of HA-Sec4p, HA-Ypt1p, and Sro7p was then induced by the addition of 2% galactose for 45 min. Cell pellets were resuspended in immunoprecipitation buffer (20 mM Hepes, pH 7.2, 150 mM KCl, 1 mM DTT, 6 mM MgCl2, and 1 mM EDTA) containing 1 mM GTP, 1% NP-40, and protease inhibitors (1 mM PMSF, 2 μg/ml pepstatin A, 2 μg/ml chymostatin, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 μg/ml antipain). 2 g zirconia-silica beads were added, and the cells were lysed by homogenization in a Mini-Bead Beater (Biospec Products). Unbroken cells and debris were eliminated by centrifugation at 10,000 g for 20 min at 4°C. The concentration of the yeast extracts was adjusted to 1 mg/ml, 30 μl of 50% protein G–Sepharose beads were added to 500 μl of extract, and the reaction was incubated for 90 min at 4°C. Beads were pelleted, 7.5 μl rat α-HA antibody was added to the supernatants, and the reactions were incubated overnight at 4°C. 6 μl of 50% protein G–Sepharose beads were added and incubated for another 30 min. The beads were pelleted, washed several times with immunoprecipitation buffer, and bound proteins were subjected to Western blot analysis.
Invertase secretion
The monitoring of invertase secretion was performed as described previously (Wiederkehr et al., 2003).
Image analysis
Data were digitalized using a scanner (HP Scanjet 4570c; Hewlett Packard).
Online supplemental material
Fig. S1 shows the growth of exocytic temperature-sensitive mutants combined with the deletion of SRO7 compared with the single mutants and wild-type yeast at 24 and 14°C (data are summarized in Table II). Fig. S2 shows that the overexpression of SEC4 and SRO7 suppresses the growth phenotype of a sec15-1 mutant strain (A) but not the deletion of SEC15 (B). Fig. S3 shows that the overexpression of single exocyst subunits does not suppress the salt sensitivity of an sro7Δ strain.
Supplementary Material
Acknowledgments
We thank Martina Medkova and Johan-Owen De Craene for advice during this study and Helge Grosshans for critical reading of the manuscript. We also thank the Horwich and Koelle/Solomon/Steitz laboratories for sharing their equipment.
This work was supported by grants from the National Institutes of Health to P. Novick (GM 35370 and CA 46128), J. Yates III (P41 RR11823-10), and P. Brennwald (R01GM54712).
Abbreviations used in this paper: GEF, guanine nucleotide exchange factor; GDP, guanosine 5′-diphosphate; lgl, lethal giant larvae; SC, synthetic complete; YPD, yeast peptone dextrose.
References
- Adamo, J.E., G. Rossi, and P. Brennwald. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell. 10:4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, K.H. 2004. Structural and functional conservation of the lgl recessive oncogenes. Int. J. Oncol. 24:1257–1261. [PubMed] [Google Scholar]
- Bilder, D. 2004. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18:1909–1925. [DOI] [PubMed] [Google Scholar]
- Brennwald, P., B. Kearns, K. Champion, S. Keranen, V. Bankaitis, and P. Novick. 1994. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 79:245–258. [DOI] [PubMed] [Google Scholar]
- Carr, C.M., E. Grote, M. Munson, F.M. Hughson, and P.J. Novick. 1999. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis, S., and M. Zerial. 2000. Purification and identification of novel Rab effectors using affinity chromatography. Methods. 20:403–410. [DOI] [PubMed] [Google Scholar]
- Deneka, M., M. Neeft, and P. van der Sluijs. 2003. Regulation of membrane transport by rab GTPases. Crit. Rev. Biochem. Mol. Biol. 38:121–142. [DOI] [PubMed] [Google Scholar]
- Eng, J., A. McCormack, and J. Yates. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spetrom. 5:976–989. [DOI] [PubMed] [Google Scholar]
- Gangar, A., G. Rossi, A. Andreeva, R. Hales, and P. Brennwald. 2005. Structurally conserved interaction of Lgl family with SNAREs is critical to their cellular function. Curr. Biol. 15:1136–1142. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami, S., W.K. Huh, K. Bower, R.W. Howson, A. Belle, N. Dephoure, E.K. O'Shea, and J.S. Weissman. 2003. Global analysis of protein expression in yeast. Nature. 425:737–741. [DOI] [PubMed] [Google Scholar]
- Goud, B., A. Salminen, N.C. Walworth, and P.J. Novick. 1988. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 53:753–768. [DOI] [PubMed] [Google Scholar]
- Govindan, B., R. Bowser, and P. Novick. 1995. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, E., and P.J. Novick. 1999. Promiscuity in Rab-SNARE interactions. Mol. Biol. Cell. 10:4149–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., A. Grant, and P. Novick. 1999. a. Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 274:23558–23564. [DOI] [PubMed] [Google Scholar]
- Guo, W., D. Roth, C. Walch-Solimena, and P. Novick. 1999. b. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, Y., A. Gruhler, A. Heilbut, G.D. Bader, L. Moore, S.L. Adams, A. Millar, P. Taylor, K. Bennett, K. Boutilier, et al. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 415:180–183. [DOI] [PubMed] [Google Scholar]
- Huh, W.K., J.V. Falvo, L.C. Gerke, A.S. Carroll, R.W. Howson, J.S. Weissman, and E.K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature. 425:686–691. [DOI] [PubMed] [Google Scholar]
- Idrissi, F.Z., B.L. Wolf, and M.I. Geli. 2002. Cofilin, but not profilin, is required for myosin-I-induced actin polymerization and the endocytic uptake in yeast. Mol. Biol. Cell. 13:4074–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, J., A. Toh-e, and Y. Matsui. 1996. Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a rho-type small GTPase, provides evidence for a role in bud formation. Genetics. 142:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., C. Richardson, R. Litt, and N. Segev. 1995. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J. Cell Biol. 131:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami, M., A. Toh-e, and Y. Matsui. 1998. Sro7p, a Saccharomyces cerevisiae counterpart of the tumor suppressor l(2)gl protein, is related to myosins in function. Genetics. 149:1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T.S., S.L. Reck-Peterson, N.B. Elkind, M.S. Mooseker, P.J. Novick, and J.A. Cooper. 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell. 11:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, K., F. Bohl, I. Sjostrom, N. Akhtar, D. Strand, B.M. Mechler, R. Grabowski, and L. Adler. 1998. The Saccharomyces cerevisiae SOP1 and SOP2 genes, which act in cation homeostasis, can be functionally substituted by the Drosophila lethal(2)giant larvae tumor suppressor gene. J. Biol. Chem. 273:33610–33618. [DOI] [PubMed] [Google Scholar]
- Lehman, K., G. Rossi, J.E. Adamo, and P. Brennwald. 1999. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 146:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, J.P., S. Stone, Y. Jiang, P. Lyons, and S. Ferro-Novick. 1994. Ypt1p implicated in v-SNARE activation. Nature. 372:698–701. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., A. McKenzie III, D.J. Demarini, N.G. Shah, A. Wach, A. Brachat, P. Philippsen, and J.R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14:953–961. [DOI] [PubMed] [Google Scholar]
- MacCoss, M.J., W.H. McDonald, A. Saraf, R. Sadygov, J.M. Clark, J.J. Tasto, K.L. Gould, D. Wolters, M. Washburn, A. Weiss, et al. 2002. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc. Natl. Acad. Sci. USA. 99:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, Y., and E.A. Toh-e. 1992. a. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol. Cell. Biol. 12:5690–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, Y., and A. Toh-e. 1992. b. Isolation and characterization of two novel ras superfamily genes in Saccharomyces cerevisiae. Gene. 114:43–49. [DOI] [PubMed] [Google Scholar]
- Novick, P., C. Field, and R. Schekman. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 21:205–215. [DOI] [PubMed] [Google Scholar]
- Ortiz, D., M. Medkova, C. Walch-Solimena, and P. Novick. 2002. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C.Y., L. Manning, R. Albertson, and C.Q. Doe. 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 408:596–600. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487–491. [DOI] [PubMed] [Google Scholar]
- Pruyne, D.W., D.H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and P.J. Novick. 1987. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 49:527–538. [DOI] [PubMed] [Google Scholar]
- Segev, N. 2001. Ypt/Rab GTPases: regulators of protein trafficking. Sci. STKE. 10.1126/stke.2001.100.re11. [DOI] [PubMed]
- Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3–21. [DOI] [PubMed] [Google Scholar]
- Sogaard, M., K. Tani, R.R. Ye, S. Geromanos, P. Tempst, T. Kirchhausen, J.E. Rothman, and T. Sollner. 1994. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 78:937–948. [DOI] [PubMed] [Google Scholar]
- Strand, D., R. Jakobs, G. Merdes, B. Neumann, A. Kalmes, H.W. Heid, I. Husmann, and B.M. Mechler. 1994. The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J. Cell Biol. 127:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb, D.L., W.H. McDonald, and J.R. Yates III. 2002. DTASelect and contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D.R., T. Maurice, D. Roth, and P. Novick. 1996. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Toikkanen, J.H., K.J. Miller, H. Soderlund, J. Jantti, and S. Keranen. 2003. The beta subunit of the Sec61p endoplasmic reticulum translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J. Biol. Chem. 278:20946–20953. [DOI] [PubMed] [Google Scholar]
- Wagner, W., P. Bielli, S. Wacha, and A. Ragnini-Wilson. 2002. Mlc1p promotes septum closure during cytokinesis via the IQ motifs of the vesicle motor Myo2p. EMBO J. 21:6397–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., R.N. Collins, and P.J. Novick. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., M. Sacher, and S. Ferro-Novick. 2000. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J. Cell Biol. 151:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr, A., Y. Du, M. Pypaert, S. Ferro-Novick, and P. Novick. 2003. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell. 14:4770–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr, A., J.O. De Craene, S. Ferro-Novick, and P. Novick. 2004. Functional specialization within a vesicle-tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J. Cell Biol. 167:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz, A. 2000. Tumor suppressors: linking cell polarity and growth control. Curr. Biol. 10:R624–R626. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]
- Zhang, X., P. Wang, A. Gangar, J. Zhang, P. Brennwald, D. TerBush, and W. Guo. 2005. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 170:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.