Abstract
In metazoans, γ-tubulin acts within two main complexes, γ-tubulin small complexes (γ-TuSCs) and γ-tubulin ring complexes (γ-TuRCs). In higher eukaryotes, it is assumed that microtubule nucleation at the centrosome depends on γ-TuRCs, but the role of γ-TuRC components remains undefined.
For the first time, we analyzed the function of all four γ-TuRC–specific subunits in Drosophila melanogaster: Dgrip75, Dgrip128, Dgrip163, and Dgp71WD. Grip-motif proteins, but not Dgp71WD, appear to be required for γ-TuRC assembly. Individual depletion of γ-TuRC components, in cultured cells and in vivo, induces mitotic delay and abnormal spindles. Surprisingly, γ-TuSCs are recruited to the centrosomes. These defects are less severe than those resulting from the inhibition of γ-TuSC components and do not appear critical for viability. Simultaneous cosilencing of all γ-TuRC proteins leads to stronger phenotypes and partial recruitment of γ-TuSC. In conclusion, γ-TuRCs are required for assembly of fully functional spindles, but we suggest that γ-TuSC could be targeted to the centrosomes, which is where basic microtubule assembly activities are maintained.
Introduction
In metazoans, the centrosome organizes the microtubule cytoskeleton. The molecular mechanisms responsible for the initiation and the regulation of microtubule assembly remain unclear, although γ-tubulin appears critical to these processes. In addition to a centrosomal fraction, γ-tubulin is present in cytosolic high-order protein structures (Akashi et al., 1997; Murphy et al., 1998; Oegema et al., 1999; Fujita et al., 2002). In Drosophila melanogaster, two main complexes have been characterized. The simplest ones, called γ-tubulin small complexes (γ-TuSCs), are salt-stable tetramers of ∼10S that are composed of two γ-tubulin molecules and two associated proteins, Dgrip84 and Dgrip91 (Oegema et al., 1999). They represent the basic components of the larger complexes, the γ-tubulin ring complexes (γ-TuRCs). γ-TuRCs, whose sedimentation coefficients range from 25 to 35S, contain at least four other proteins (Dgrip75, Dgrip128, Dgrip163, and Dgp71WD) in addition to the γ-TuSC subunits, in a yet unknown stoichiometry. Dgrip75, Dgrip128, and Dgrip163 exhibit sequence homologies called grip motifs, with the two γ-tubulin–associated proteins of the γ-TuSC (Fava et al., 1999; Gunawardane et al., 2000; Murphy et al., 2001). In contrast, Dgp71WD does not posses any grip motifs, but contains seven WD (tryptophan–aspartic acid) repeats (Haren et al., 2006). In vitro, this protein directly interacts with the grip motif containing γ-TuRC subunits, suggesting that it may play a scaffolding role in γ-TuRC organization (Gunawardane et al., 2003). Current models suggest that γ-TuRCs that have been previously assembled in the cytoplasm are recruited to the centrosomes, where they play a role in microtubule nucleation and stabilization (Stearns and Kirschner, 1994; Zheng et al., 1995; Fava et al., 1999; Zhang et al., 2000).
The functions of γ-tubulin and its associated proteins in the γ-TuSC have been extensively studied. Deletion of either corresponding gene is lethal, resulting in an accumulation of cells in mitosis. This is usually correlated with the appearance of strong mitotic defects such as monopolar structures or bipolar spindles exhibiting unfocused poles, impairment of pole maturation, and increase in aneuploidy (Oakley et al., 1990; Sunkel et al., 1995; Geissler et al., 1996; Spang et al., 1996; Knop et al., 1997; Barbosa et al., 2000; Paluh et al., 2000; Vardy and Toda, 2000; Hannak et al., 2002; Colombie et al., 2006). In contrast, our knowledge about the function of γ-TuRC–specific components is limited. In D. melanogaster, Dgrip75 is essential for fertility, but not for viability. Dgrip75 loss-of-function mutants specifically affect localization of the maternal determinant bicoid during oogenesis, suggesting a role in the organization or in the dynamics of a subset of microtubules (Schnorrer et al., 2002). In Schizosaccharomyces pombe, mutants in Gfh1 (a Dgrip75 orthologue) and in Alp16 (a Dgrip163 orthologue) exhibit defects associated with altered microtubule function, but without any effect on cell viability (Fujita et al., 2002; Venkatram et al., 2004). Current models cannot explain these data. Instead, they raise questions not only about the redundancy or the specificity of the γ-TuRC–specific proteins but also about the respective functions of γ-TuSCs and γ-TuRCs. In this work, we have tested whether γ-tubulin is only recruited to the centrosome in the form of γ-TuRCs or if γ-TuSCs can be recruited and subsequently matured into functional γ-tubulin complexes by attracting additional components. To this aim, we have developed two strategies: (1) RNA interference (RNAi) in cultured cells involving individual or concomitant depletion of γ-TuRC components, and (2) genetic analyses by taking advantage of the availability of mutant strains (Dgrip75, Dgrip163, and Dgp71WD). We demonstrate that the γ-TuRC–specific subunits display functional specificities and that the γ-TuSCs could be targeted to the centrosome where basic microtubule assembly functions are maintained.
Results
RNAi-directed depletion of Dgrip75 impairs cytosolic γ-TuRC assembly or stability
First, we characterized the consequences of the depletion of a γ-TuRC–specific protein on the assembly of cytosolic γ-tubulin complexes. Cultured D. melanogaster S2 cells were treated by RNAi to deplete Dgrip75, a grip protein specifically present in the γ-TuRC (Fava et al., 1999). The treatment led to a strong decrease of the protein level (Fig. 1 A; >95% of the control level, as judged by Western blot analysis). This effect was specific, as determined by examining the amount of the three γ-TuSC proteins (γ-tubulin, Dgrip84, and Dgrip91) and actin (Fig. 1, A and D). Immunofluorescence analysis of control cells showed that although Dgrip75 was undetectable at the interphase centrosome, it localized to the poles at the onset of mitosis, where it was maintained throughout cell division (Fig. 1 B, c). In marked contrast, the protein was absent from the mitotic centrosomes in Dgrip75-depleted cells, consistent with Western blot quantification (Fig. 1 B, d; and Table I). When extracts from treated cells were submitted to sucrose gradient sedimentation, γ-TuRCs were severely reduced, as indicated by immunoblotting of soluble fractions with antibodies against γ-tubulin, Dgrip84, Dgrip91, Dgrip128, and Dgrip163 (Fig. 1 C). The main remaining complexes appeared to be γ-TuSCs, as judged by their protein content and their sedimentation coefficient. In addition, the total level, as well as the soluble and cytoskeletal fractions of the three γ-TuSC proteins, are unchanged in control and Dgrip75-depleted cells (Fig. 1 D), suggesting a redistribution of these proteins in the different complexes rather than a change in quantity. In contrast, after Dgrip75-RNAi treatment, we noticed a decrease in the total level of the two other grip-motif proteins of the γ-TuRC, Dgrip128 and Dgrip163. The remaining Dgrip128 protein was distributed on the gradient in the form of heterogeneous and uncharacterized complexes with apparent masses equal or slightly higher than the mass of the γ-TuSC. Dgrip163 protein migrated mainly in light fractions (<10S). One hypothesis could be that this protein was present as a monomeric or dimeric form. Thus, Dgrip75 appears to be required for efficient assembly or stability of cytoplasmic γ-TuRCs.
Figure 1.
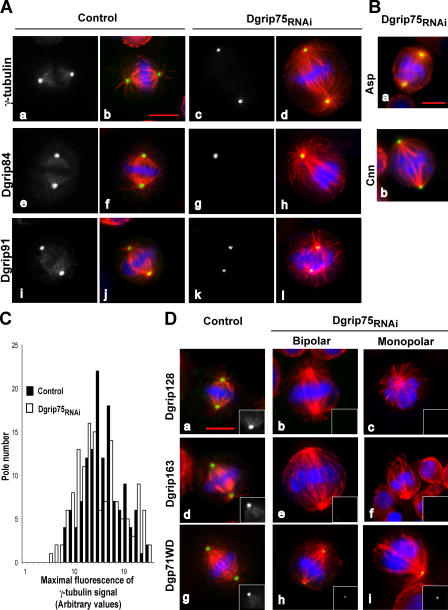
Characterization of Dgrip75 depletion by RNAi. (A) Analysis of protein depletion by Western blot. Total protein extracts of control or treated cells (Dgrip75RNAi) were analyzed using Dgrip75 affinity–purified antibodies and antibodies against Dgrip84, γ-tubulin, or actin (internal loading control). (B) Analysis of protein depletion by immunofluorescence. a and b, microtubules; c and d, Dgrip75 signal. A quantitative view is shown in Table I. (C) Analysis of γ-tubulin complexes after Dgrip75 depletion. Total protein extracts prepared from control or treated cells (Dgrip75RNAi) were centrifuged in a sucrose gradient. Soluble fractions were analyzed by immunoblotting against γ-TuRC components. (D) Recruitment of γ-TuSC components into the cytoskeletal fraction after Dgrip75 depletion. Total protein extracts (T) of control or treated cells (Dgrip75RNAi) were centrifuged for 10 min at 10,000 g. Then, the supernatants (S) and the pellets (P) were analyzed by immunoblotting with antibodies against Dgrip91, Dgrip84, γ-tubulin, and Dgrip75. Bar, 5 μm.
Table I.
Accumulation of centrosomal components in mitotic cells after Dgrip75 depletion in cultured cells
| Staining | Control | Dgrip75RNAi
|
|
|---|---|---|---|
| Bipolar | Monopolar | ||
| % | % | % | |
| γ-Tubulin | 100 (n = 100) | 100 (n = 124) | 95 (n = 66) |
| Dgrip84 | 99 (n = 186) | 98 (n = 170) | 98 (n = 50) |
| Dgrip91 | 91 (n = 103) | 100 (n = 55) | 100 (n = 48) |
| Dgrip75 | 100 (n = 95) | 6 (n = 70) | 0 (n = 52) |
| Dgrip128 | 78 (n = 71) | 3 (n = 93) | nd |
| Dgrip163 | 98 (n = 132) | 24 (n = 119) | nd |
| Dgp71WD | 95 (n = 109) | 100 (n = 65) | 100 (n = 43) |
Microtubules were stained with antibodies against α-tubulin and chromosomes with DAPI, whereas the spindle poles were immunostained with antibodies against γ-tubulin, Dgrip84, Dgrip91, Dgrip75, Dgrip128, Dgrip163, and Dgp71WD. For a correct interpretation, we need to keep in mind a decrease in the total amount of Dgrip128 and Dgrip163 in Dgrip75-depleted cells. n, number of prometaphases/metaphases analyzed. nd, not determined.
Impairment of γ-TuRC assembly induces moderate mitotic defects, but does not preclude γ-TuSC centrosomal recruitment
We next investigated whether Dgrip75 depletion, and thus the subsequent decrease of γ-TuRCs, affected mitotic progression. The mitotic index was significantly increased by ∼2.6-fold (2.9%; n = 4,766; probability of 95% [P95] = 2.5–3.3 in treated cells, compared with 1.1%; n = 9,235; P95 = 0.9–1.3 in control cells). This mitotic accumulation coincided with the maintenance of an active mitotic checkpoint, as judged by a strong BubR1 signal at kinetochores (not depicted), which was indicative of a transient block in prometaphase. α-Tubulin immunostaining analysis confirmed an accumulation of cells in premetaphase stages (increased 1.8-fold in frequency), whereas postmetaphase figures exhibited a 2.6-fold decrease relative to controls (Table II). Aberrant mitotic figures were observed, mainly monopolar spindles and bipolar spindles that were either elongated or with lagging chromosomes (Fig. 2 and Table II). Approximately one third of prometaphases and metaphases were still organized as bipolar structures, albeit with a longer interpolar distance (average increase of 50%; 7.9 ± 0.9 in treated cells vs. 5.3 ± 0.7 in controls) and a poor microtubule density. However, treated bipolar spindles exhibited astral microtubules (Fig. 2 A, d, h, and l). Their poles were normally focused, consistent with polar localization of Asp (Fig. 2 B, a), a marker of the spindle microtubules minus ends (Wakefield et al., 2001). They seemed to properly separate their centrosome, as 97% (n = 112; P95 = 92–99) exhibited centrosomin (Cnn) labeling at both poles (Fig. 2 B, b; Megraw et al., 1999). Surprisingly, in Dgrip75-depleted cells, γ-tubulin was still recruited to the poles at all stages of mitosis (Fig. 2 A, a–d; and Table I). The maximal fluorescence values raised by γ-tubulin antibodies at the two poles of symmetrical spindles did not differ between Dgrip75-treated and control cells (Fig. 2 C). The two γ-tubulin partners in the γ-TuSC, Dgrip84, and Dgrip91 were also targeted to the poles in an efficient manner (Fig. 2 A, e–l; and Table I). At the same time, pole localization of γ-TuRC–specific proteins (Dgrip128, Dgrip163, and Dgp71WD) was significantly inhibited, but not completely abolished (Fig. 2 D and Table I). The reductions in Dgrip128 or Dgrip163 stainings (Fig. 2 D, a–f, Table I) may result, at least partly, in the overall decrease in the levels of these proteins after Dgrip75 depletion. Dgp71WD staining was present, but reduced in intensity in most of the cases (Fig. 2 D, g–i, insets). To illustrate these changes, we performed double-labeling immunofluorescence on cells depleted of Dgrip75, showing colocalization of γ-tubulin with γ-TuSC–associated proteins (Dgrip84 or Dgrip91) or with Dgp71WD, whereas the γ-TuRC proteins Dgrip128 or Dgrip163 were no longer detectable (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1). The localization of γ-tubulin along spindle microtubules, observed in 97% (n = 138; P95 = 93–99) of control cells, was detected in only 10% (n = 146; P95 = 5–15) of the depleted cells (Fig. 2 A, a–d). Similarly, the two γ-TuSC proteins, Dgrip84 and Dgrip91, were no longer detected along spindle microtubules (Fig. 2 A, e–l). Moreover, staining of γ-TuSC proteins at the midbody was impaired in treated cells (not depicted). Hence, the assembly of cytoplasmic γ-TuRCs does not appear as a prerequisite for centrosomal recruitment of the γ-TuSCs, but seems essential for its localization along spindle microtubules and at the midbody.
Table II.
Distribution of the different mitotic stages after Dgrip75 depletion in cultured cells
| Mitotic figures | Control n = 100 |
Dgrip75RNAi
n = 244 |
|---|---|---|
| % | % | |
| Unfocuseda | 21 ± 8b | 24 ± 6 |
| Prometaphases/metaphases | ||
| Symmetrical bipolar | 28 ± 9 | 20 ± 5 |
| Abnormal bipolar | 4 ± 4 | 22 ± 6 |
| Monopolar | 0 | 16 ± 4 |
| Postmetaphase stages | 47 ± 10 | 18 ± 4 |
Mitotic stages were determined after chromosome staining (DAPI) and immunolabeling of microtubules (α-tubulin antibodies). n, number of mitotic spindles analyzed. These results are representative of three independent experiments.
Spindles with no evident polarity, which represent mainly prophase stages or highly abnormal spindles.
Confidence intervals calculated for P95.
Figure 2.
Immunofluorescence analysis of phenotypes induced by Dgrip75 depletion in S2 cells. (A) Localization of γ-TuSC components in mitotic cells. Control or Dgrip75-treated cells (Dgrip75RNAi) were analyzed by staining with antibodies against γ-tubulin (a and c), Dgrip84 (e and g), and Dgrip91 (i and k). Merge (b, d, f, h, j, and l): blue, chromosomes; red, microtubules; green, γ-TuSC components. (B) Spindle pole focusing and localization of centrosomal markers. Dgrip75-depleted cells were stained with antibodies against Asp (a, green) or Cnn (b, green). Red, α-tubulin; blue, chromosomes. (C) Quantification of polar γ-tubulin by immunofluorescence. The maximal signal obtained after γ-tubulin staining was scored on each pole of bipolar spindles for control (closed bars) and Dgrip75-depleted cells (open bars). Correlations of 0.73 (n = 67; P95 = 0.62–0.84) and 0.72 (n = 69; P95 = 0.61–0.83), respectively, were observed between the maximal fluorescence measured at the two poles of control or treated cells. (D) Localization of γ-TuRC–specific components (Dgrip128, Dgrip163, and Dgp71WD) in mitotic cells. Blue, chromosomes; red, microtubules; green, γ-TuRC–specific components. Insets represent a hemispindle stained with antibodies against Dgrip128, Dgrip163, and Dgp71WD. For A and D, a quantitative view is shown in Table I. Bars, 5 μm.
Dgrip75 loss-of-function mutant is delayed in mitosis, but still recruits γ-tubulin to the centrosome
To examine the functional significance of Dgrip75 in vivo, we took advantage of the availability of the mutant allele 175–14 (Dgrip75175), which was either a null or a strong allele (Schnorrer et al., 2002). Dgrip75175 mutant was viable, although adults of both sexes exhibited a slight increase in lethality some days after hatching. Moreover, they showed abnormalities in the abdominal cuticle segmentation and the thoracic macrochaete pattern (unpublished data), which were common in mutations affecting mitosis. Finally, mutant females were sterile because of a failure to undergo normal oogenesis.
Although Dgrip75 did not appear essential for viability, the phenotypes observed after RNAi treatment of cultured cells prompted us to study the mitotic processes in mutant L3 larval brains. Western blot analysis showed that Dgrip75 levels were reduced below the threshold of detection in Dgrip75175 brain extracts (Fig. 3 A). The mitotic index was elevated approximately three-to fourfold (2.5%; n = 12,410; P95 = 2.2–2.8) compared with wild-type cells (0.7%; n = 16,134; P95 = 0.6–0.8). Mutant cells accumulated in prometaphase stages, whereas postmetaphase figures were reduced (unpublished data). More than half of the mutant cells exhibited overcondensed chromosomes (60%; n = 186; P95 = 53–67) compared with wild type (2%; n = 211; P95 = 0–4; Fig. 3 B). These results were consistent with prolonged prometaphase and metaphase stages. However, only very few mutant cells showed an aneuploid phenotype (6%; n = 112; P95 = 3–9) compared with wild type (1%; n = 116; P95 = 0–3), and this low frequency was consistent with Dgrip75175 adult viability.
Figure 3.
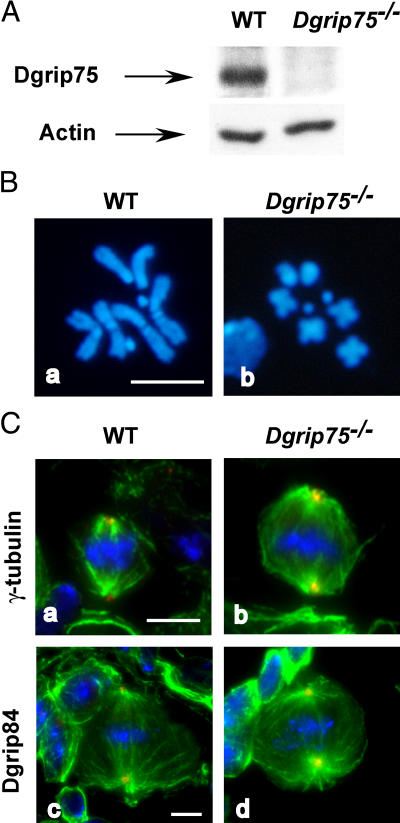
In vivo phenotypes observed in Dgrip75 mutant brains. (A) Characterization of Dgrip75 depletion by Western blot. Total protein extracts from wild-type (WT) or Dgrip75 mutant (Dgrip75 −/−) L3 larval brains were analyzed with affinity-purified Dgrip75 antibodies. Actin was used as an internal loading control. (B) Analysis of chromosomal figures. Wild-type or mutant L3 larval brains were stained with DAPI. Five brains were scored for each genotype. (C) Immunofluorescence analysis of the mitotic figures in Dgrip75 mutant neuroblasts. Green, spindle; blue, chromosomes; red, γ-tubulin (a and b) and Dgrip84 (c and d). Bars, 5 μm.
Transient prometaphase delays observed in the Dgrip75 mutant could be a consequence of defects in the mitotic apparatus. Nevertheless, immunofluorescence analysis revealed neither a clear difference in spindle morphology nor a significant decrease of cells stained positively for γ-tubulin or Dgrip84 (Fig. 3 C). Given the limitations of immunofluorescence in neuroblasts, we cannot exclude defects in microtubule organization, such as changes in microtubule density or interpolar distance.
These data suggest that in Dgrip75175 brains, most of the cells complete cell division even though mitosis might be slowed down, consistent with our observations in RNAi-treated cultured cells. γ-TuSC components are still recruited to the centrosomes and most of the mitotic apparatus are able to ensure correct chromosome segregation.
Removal of Dgrip128 or Dgrip163 is not essential for centrosomal γ-TuSC recruitment
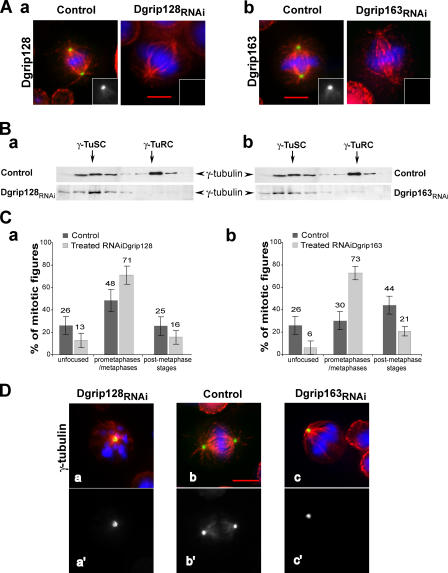
To evaluate whether the phenotypes induced by Dgrip75 depletion were specific of this grip-motif protein, we performed an RNAi treatment against Dgrip128 or Dgrip163, the two other grip-motif γ-TuRC–specific components. Depletion was efficient in either case: after Dgrip128 RNAi, no poles (n = 142) were labeled, and after Dgrip163 RNAi, <4% of the poles (n = 150) were labeled with respective Dgrip128 and Dgrip163 antibodies (Fig. 4 A). Western blot analysis confirmed this view (not depicted). Sucrose gradient analysis of extracts depleted for Dgrip128 or Dgrip163 showed a marked decrease in γ-TuRC content (Fig. 4 B). These experiments show that these proteins, like Dgrip75, appear involved in the assembly or stability of γ-TuRCs. Moreover, silencing of either protein led to accumulation of cells in mitosis (approximately three- to fourfold), which resulted from a higher frequency of prometaphases concomitant with a decrease in postmetaphase stages (Fig. 4 C). Mitotic phenotypes were mainly characterized by elongated bipolar spindles and less frequently by monopolar structures. γ-Tubulin (Fig. 4 D) as well as Dgrip84 and Dgrip91 (not depicted) were efficiently recruited to the centrosomes, even in mitotic cells that exhibited severe phenotypes (i.e., monopolar spindles). However, γ-tubulin labeling along spindle microtubules was no longer observed (Fig. 4 D). It was striking that these phenotypes were similar to the defects obtained after depletion of Dgrip75.
Figure 4.
Phenotypes observed in Dgrip128- or Dgrip163-depleted cells. For morphological phenotype analyses, cells were immunostained against microtubules (red) and chromosomes (blue). (A) Analysis of Dgrip128 (a) or Dgrip163 (b) depletions. Dgrip128 (a) or Dgrip163 (b) are shown in green. Insets represent a hemispindle stained with antibodies against Dgrip128 and Dgrip163. (B) Pattern of γ-tubulin cytosolic complexes after Dgrip128 (a) or Dgrip163 (b) depletions. Soluble fractions from a sucrose gradient were analyzed by immunoblotting against γ-tubulin. (C) Distribution of cells in the different stages of mitosis after depletion of Dgrip128 (a) or Dgrip163 (b). The different mitotic stages were scored over >100 cells undergoing mitosis. Error bars represent the confidence interval calculated for P95. (D) Analysis of localization of γ-tubulin in mitotic cells. γ-Tubulin is stained in green in merge (a–c) and in white in a′–c′. More than 97% of Dgrip128- (a) or Dgrip163-depleted (c) cells showed γ-tubulin at the poles (scored on >100 cells in the prometaphase/metaphase stages). Bars, 5 μm.
Furthermore, we next performed an in vivo study using a Dgrip163 P insertion mutant (Dgrip163GE27087) in which the P transposable element was inserted into exon 4 (out of 5) between codons 822 and 823. This mutant behaved genetically as a null or strong allele (unpublished data). Dgrip163 mutants exhibited reduced viability. The emerging homozygous or hemizygous Dgrip163GE27087 adults displayed abdominal abnormalities, and mutant females were sterile. Altogether, these studies reveal that inhibition of each individual grip-motif protein specific of the γ-TuRC leads to similar phenotypes in cultured cells and in vivo.
Dgp71WD is neither essential for γ-TuRC assembly nor for its recruitment to the centrosome
In contrast to the other proteins specific of the γ-TuRCs, Dgp71WD does not belong to the same structural family (Gunawardane et al., 2003). RNAi treatment induced a strong inhibition, as judged by Western blot analysis (Fig. 5 A). In agreement with this quantification, Dgp71WD centrosomal recruitment was severely impaired in treated cells (1%; n = 385; P95 = 0–2) compared with control cells (95%; n = 296; P95 = 93–97; Fig. 5 B). In treated cells, we noticed the systematic loss of Dgp71WD staining on other mitotic structures, such as spindle microtubules (Fig. 5 B, inset) and midbodies (not depicted). As Dgp71WD contains WD repeats and interacts in vitro with the four grip-motif subunits Dgrip84, Dgrip91, Dgrip128, and Dgrip163 (Gunawardane et al., 2003), it might play a role as a scaffold for cytosolic γ-TuRC assembly. When control extracts were subjected to sucrose gradient sedimentation, this protein appeared not only as part of γ-TuRCs but also of smaller uncharacterized complexes (Fig. 5 C). In Dgp71WD-depleted conditions (Fig. 5 C), no significant modification in the quantity of ∼30S complexes is observed, as judged by γ-tubulin or Dgrip75 labeling. This result suggests that Dgp71WD, in contrast to Dgrip75, appears to be dispensable for the assembly or the stability of large γ-tubulin complexes. However, we cannot exclude the possibility that the assembly of these complexes is mediated by residual amounts of Dgp71WD.
Figure 5.
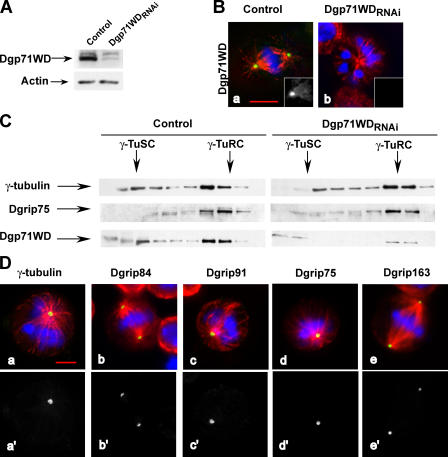
Phenotypes induced by Dgp71WD depletion in cells. (A) Analysis of Dgp71WD depletion by Western blot. Total protein extracts of control or of treated cells (Dgp71WDRNAi) were analyzed using antibodies against Dgp71WD or actin (internal loading control). (B) Analysis of the protein depletion by immunofluorescence. Control (a) or Dgp71WD-treated cells (b) were analyzed by staining with antibodies against Dgp71WD (insets). Merge: blue, chromosomes; red, microtubules; green, Dgp71WD. (C) Pattern of γ-tubulin cytosolic complexes after Dgp71WD depletion. Soluble fractions from a 5–40% sucrose gradient were analyzed by immunoblotting against γ-tubulin, Dgrip75, or Dgp71WD. (D) Localization of γ-tubulin, Dgrip84, Dgrip91, Dgrip75, and Dgrip163 in mitotic Dgp71WD-depleted cells. γ-TuRC components are shown in green (a–e) or in white (a′–e′). For D, a quantitative view is shown in Fig. S2 C, available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1. Bars, 5 μm.
Studies of the mitotic phenotypes observed after Dgp71WD down-regulation revealed similarities to the defects recorded after the removal of Dgrip75, Dgrip128, or Dgrip163; an increase of the mitotic index (2.7%; n = 7,970; P95 = 2.3–3.1) compared with control cells (0.9%; n = 19,650; P95 = 0.8–1); and an accumulation of cells in the prometaphase and metaphase stages (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1). Dgp71WD-depleted cells showed defects in spindle morphology, such as bipolar spindles with unfocused poles or monopolar structures (Fig. 5, B and D; and Fig. S2 B) with unseparated poles, as determined by Cnn staining (not depicted). γ-TuSC components (γ-tubulin, Dgrip84, and Dgrip91), as well as γ-TuRC–specific subunits (Dgrip75 and Dgrip163), were still targeted to the mitotic centrosomes, but often the stainings were weak (Fig. 5 D and Fig. S2 C). These proteins were no longer detected at the spindles. These results suggest that Dgp71WD is dispensable for γ-TuRC assembly and recruitment, but is required for the efficient function of this complex.
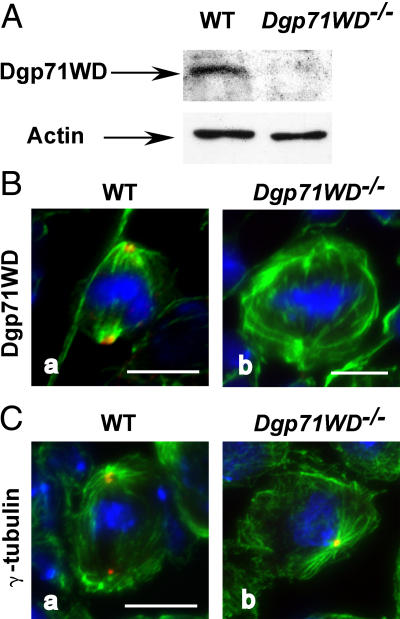
A genetic approach was performed to test this hypothesis in vivo. We used a mutant strain that carried a P element insertion (GE30807) in the 5′ untranslated mRNA region at position 49 from the translation initiator ATG. As shown by Western blot, the level of Dgp71WD was strongly reduced in a mutant larval brain extract compared with a wild-type extract (Fig. 6 A). An immunofluorescence analysis performed in larval brains sustained that Dgp71WD was no longer detectable in mutants (n = 120), whereas 96% of wild-type poles (n = 110; P95 = 92–100) exhibited a clear staining (Fig. 6 B). These results indicate that Dgp71WDGE30807 is at least a strong allele. This was confirmed by analysis of hemizygous flies, using a chromosomal deficiency that covers Dgp71WD. Most of the homozygous or hemizygous mutants reached the adult stage. However, they showed a shorter life, morphological abnormalities, and female sterility. Mitotic phenotypes had been analyzed in L3 larval brains confirming RNAi-mediated phenotypes. We observed a fourfold increase in the mitotic index, an accumulation in prometaphase stages associated with a significant hypercondensation of chromosomes and a high incidence of disorganized or monopolar spindles (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1). However, γ-tubulin is detected at all the centrosomes of Dgp71WDGE30807 neuroblasts (n = 130; Fig. 6 C). Moreover, mutant neuroblasts exhibited an increase in aneuploidy (12%; n = 139; P95 = 7–17) versus wild-type brains (1%; n = 125; P95 = 0–5), suggesting that spindles without Dgp71WD were not fully functional.
Figure 6.
In vivo phenotypes observed in Dgp71WD mutant brains. (A) Analysis of Dgp71WD depletion in mutant brains by Western blot. Wild-type or Dgp71WD mutant (Dgp71WD −/−) brains were analyzed with Dgp71WD antibodies or actin (internal loading control). (B) Organization of spindles in mutant neuroblasts. (C) Localization of γ-tubulin to the spindle poles of mutant neuroblasts. green, microtubules; blue, chromosomes; red, Dgp71WD (B) or γ-tubulin (C). Bars, 5 μm.
γ-TuSC components are targeted to the poles even after cosilencing of the four γ-TuRC subunits
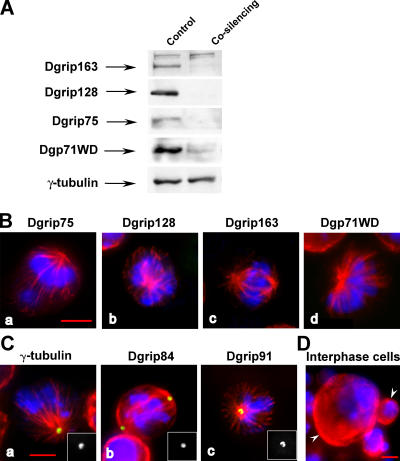
Whatever their role in the assembly and recruitment of cytosolic γ-tubulin complexes, the inhibition of each individual γ-TuRC subunit allowed efficient γ-TuSC anchoring to the centrosomes. It was possible that some grip-motif proteins were in part redundant and could complement each other to some extent. Alternatively, the residual γ-TuRC–specific proteins could account for the formation of a γ-TuRC–like structure that was unstable in the cytoplasm, but stabilized upon assembly with the pericentriolar matrix. Hence, we performed cosilencing of the four proteins Dgrip75, Dgrip128, Dgrip163, and Dgp71WD. The levels of the four proteins were dramatically reduced as judged by Western blot (Fig. 7 A) and immunofluorescence analyses (Fig. 7 B and Table III). The mitotic index was increased in treated cells (3.9%; n = 2,552; P95 = 3.4–4.4) compared with control cells (1.1%; n = 8,567; P95 = 0.8–1.4). Most of the mitotic structures consisted of monopolar prometaphases/metaphases (70%; n = 80; P95 = 60–80) compared with control cells (1%; n = 104; P95 = 0–3; Fig. 7, B and C). A striking feature of this treatment was the significant accumulation of polyploid cells and the appearance of very large interphase cells (9%; n = 281; P95 = 5–12), which appeared to account for only 0.6% of control interphase cells (n = 320; P95 = 0–1.4) (Fig. 7 D, left arrowhead). Most of the poles exhibited γ-tubulin, Dgrip84, and Dgrip91 stainings (Fig. 7 C and Table III), but at reduced intensities. Spindle labeling was no longer detectable (Fig. 7 C). These parameters showed that codepletion of these proteins induced more severe phenotypes than after any individual down-regulation. This result was supported by the synthetic lethality observed when the Dgp71WDGE30807- and Dgrip163GE27087-viable alleles were combined in the same genotype (unpublished data). Altogether, these data confirm that the γ-TuSC assembled in the cytoplasm can, at least to some extent, be directly targeted to the poles.
Figure 7.
Phenotypes observed after cosilencing of all four specific components of the γ-TuRC. (A) Analysis of the codepletion by Western blot. Total proteins extracts of control or cosilenced cells were analyzed using antibodies against Dgrip163, Dgrip128, Dgrip75, Dgp71WD, or γ-tubulin (internal loading control). (B) Analysis of the codepletion by immunofluorescence. (C) Localization of γ-TuSC components in mitotically treated cells. Insets represent a hemispindle stained with antibodies against γ-tubulin, Dgrip84, and Dgrip91. (D) Interphasic phenotype observed after cosilencing. The left arrowhead indicates a large polyploid cell, whereas the right one shows a normal sized interphase cell. (B–D) Blue, chromosomes; red, microtubules; green, γ-TuRC components (B) or γ-TuSC components (C). For B and C, a quantitative view is shown in Table III. Bars, 5 μm.
Table III.
Accumulation of centrosomal components in mitotic cells after cosilencing of Dgrip75, Dgrip128, Dgrip163, and Dgp71WD in cultured cells
| Staining | Control | Cosilencing
|
|
|---|---|---|---|
| Bipolar | Monopolar | ||
| % | % | % | |
| γ-Tubulin | 100 (n = 22) | 100 (n = 88) | 100 (n = 102) |
| Dgrip84 | 98 ± 4a | 99 ± 3 | 100 (n = 76) |
| Dgrip91 | 98 ± 4 | 100 (n = 112) | 98 ± 4 |
| Dgrip75 | 90 ± 6 | 14 ± 6 | 0 (n = 70) |
| Dgrip128 | 88 ± 6 | 2 ± 2 | 0 (n = 62) |
| Dgrip163 | 94 ± 5 | 0 (n = 61) | 0 (n = 50) |
| Dgp71WD | 95 ± 2 | 0 (n = 45) | 4 ± 6 |
Microtubules were stained with antibodies against α-tubulin and chromosomes with DAPI, whereas the spindle poles were immunostained with antibodies against γ-tubulin, Dgrip84, Dgrip91, Dgrip75, Dgrip128, Dgrip163, and Dgp71WD. For a correct interpretation, we need to keep in mind a decrease in the total amount of γTuRC–specific components in-depleted cells. n, number of prometaphases/metaphases analyzed.
Confidence intervals calculated for P95.
Discussion
The molecular mechanisms responsible for γ-tubulin recruitment to nucleation centers and microtubule nucleation itself remain poorly understood. Several models have been proposed, and all imply that the cytosolic assembly of γ-TuRCs is a critical step for γ-tubulin targeting to the centrosome. For the first time, we analyzed the function of all four γ-TuRC–specific subunits during mitosis, using either RNAi treatment in cultured cells or genetic analyses in D. melanogaster.
γ-TuRC–specific proteins play a distinct role in γ-TuRC assembly
Individual depletion of Dgrip75, Dgrip128, or Dgrip163 induced a strong decrease in cytoplasmic γ-TuRCs in cultured S2 cells. This is consistent with previous data obtained in vitro showing that immunodepletion of Xgrip210, the Xenopus laevis orthologue of Dgrip163, blocks the reassembly of purified and salt-treated γ-TuRCs (Zhang et al., 2000). In contrast to the strong decrease of γ-TuRCs observed after the depletion of grip-motif proteins, the down-regulation of Dgp71WD did not produce a significant effect on the ratio and the sedimentation coefficients of the γ-tubulin complexes. However, we cannot exclude the possibility that residual amounts of this protein are sufficient to maintain the overall structure of these complexes. Dgp71WD is probably associated with the γ- TuRCs at a low stoichiometry, explaining why no change in the sedimentation coefficient could be detected after its depletion. Our data suggest that although the grip-motif proteins are essential for the assembly and stability of γ-TuRCs, Dgp71WD appears dispensable for these processes.
The complete γ-TuRC is necessary for an efficient progression through mitosis
In Dgp71WD-depleted cells, we observed no obvious effect on the level of cytosolic γ-TuRCs and on the recruitment of the different γ-TuRC components to the mitotic centrosomes, suggesting that γ-TuRCs depleted of Dgp71WD had not completely lost their ability to be targeted to the poles. However, γ-tubulin localization along spindle microtubules was impaired and monopolar figures with poorly separated poles appeared with a high occurrence. These monopolar phenotypes are similar to those observed after depletion of Nedd1, the human Dgp71WD orthologue (Haren et al., 2006). The mitotic defects could result from a partial functionality of the γ-tubulin complexes recruited to the centrosomes or from modification in the properties of microtubule arrays that were no longer decorated by γ-tubulin. Thus, it appears that the complete γ-TuRC is required for normal mitotic progression.
γ-TuRC assembly is not a prerequisite for the centrosomal targeting of γ-tubulin
If one assumes that γ-tubulin is recruited to the centrosome in the form of γ-TuRCs, depletion of γ-TuRCs and γ-tubulin would be expected to lead to similar phenotypes. In fact, depletion of grip-motif components of the γ-TuRCs, such as Dgrip75, did not fully mimic the effects of depletion of γ-TuSC subunits on mitotic progress (Sunkel et al., 1995; Barbosa et al., 2000; Paluh et al., 2000; Vardy and Toda, 2000; Fujita et al., 2002; Venkatram et al., 2004; Colombie et al., 2006). Less severe defects were observed as indicated by the lower occurrence of monopolar spindles, the efficient distribution of centrosomes to the two poles, the maintenance of bipolar spindles with astral microtubules and focused poles. These phenotypes had been confirmed in vivo, using mutant larval neuroblasts. These moderate defects were consistent with the viability of Dgrip75 mutants and the weak percentage of aneuploidy. Surprisingly, despite a significant reduction of the cytosolic pool of γ-TuRCs, the tethering of the three γ-TuSC proteins to the centrosomes was not impaired, as judged by immunofluorescence analyses both in mutant neuroblasts and in cultured cells. Similar phenotypes were observed after individual silencing of the two other grip-motif proteins, Dgrip128 and Dgrip163. It was noteworthy that after an RNAi treatment against any one of the three grip-motif proteins specific of the γ-TuRCs, a decrease in the level of the two others was noticed (unpublished data), suggesting some coregulation between this set of proteins. Altogether, these results show that the complete γ-TuRC is not a prerequisite for the centrosomal accumulation of γ-TuSC proteins.
γ-Tubulin can be recruited to the centrosomes as γ-TuSC
Because γ-tubulin could be recruited to the centrosomes independently of the γ-TuRCs, we wanted to characterize the complex that mediates its targeting. Sucrose gradient and immunofluorescence analyses after depletion of individual γ-TuRC components strongly suggest that γ-tubulin is recruited to the centrosomes as γ-TuSC. Codepletion of the four γ-TuRC–specific proteins still allowed the polar accumulation of the γ-TuSC components in most of the cells. This latter experiment supports the hypothesis that the γ-TuSC is a vector competent for targeting γ-tubulin to the centrosome. However, as RNAi treatments do not completely remove all protein, it is possible that the residual γ-TuRC–specific proteins participate in the formation of a γ-TuRC–like structure that is unstable in the cytoplasm, but stabilized upon assembly within the pericentriolar matrix. The idea that γ-TuSC could be recruited to the poles is consistent with data reported in Saccharomyces cerevisiae, in which no orthologues of the γ-TuRC–specific proteins have been reported. In this organism, the main interactions of γ-tubulin with the microtubule-organizing centers are mediated by the two γ-tubulin–associated proteins in the γ-TuSC (Knop and Schiebel, 1997, 1998; Nguyen et al., 1998; Vinh et al., 2002). Similarly, in mammals, the γ-TuRCs can be tethered to the centrosomes via interactions of the orthologues of Dgrip84 and Dgrip91 with the centrosomal anchoring proteins kendrin and centrosome- and Golgi-localized protein kinase N–associated protein (Takahashi et al., 2002; Zimmerman et al., 2004). In D. melanogaster, the calmodulin-binding protein CP309 has been proposed to anchor γ-TuRCs to the centrosome by direct binding to the γ-TuSC (Kawaguchi and Zheng, 2004). However, γ-tubulin recruitment to the centrosomes is probably a complex process, as additional proteins, including ninein (Delgehyr et al., 2005) and centrosomin (Terada et al., 2003), provide alternative sites for γ-tubulin anchorage. Although evidence suggests that γ-tubulin can be targeted to the centrosomes in the form of γ-TuSCs, we cannot rule out that in D. melanogaster other proteins at low abundance, previously uncharacterized proteins such as the Dgrip79 and Dgrip223 identified by in silico analyses, or different combinations of known γ-tubulin partners could be involved in this recruitment (Gunawardane et al., 2003). Moreover, after concomitant depletion of all γ-TuRC–specific proteins, spindles were nonfunctional and the amount of γ-TuSC recruited to the mitotic centrosomes was reduced. Because of the observation that the small complexes are less active than γ-TuRCs in promoting nucleation (Oegema et al., 1999; Gunawardane et al., 2000), we propose that the decrease in the amount of γ-TuSCs under a threshold can be critical for spindle functionality.
Interestingly, the transient association of γ-tubulin to the spindle, as well as to the midbody (Julian et al., 1993; Lajoie-Mazenc et al., 1994; Khodjakov and Rieder, 1999; Raynaud-Messina et al., 2001, 2004), was no longer detectable after individual or concomitant RNAi treatment against Dgrip75, Dgrip128, Dgrip163, and/or Dgp71WD. Hence, mitotic γ-tubulin localization to structures outside the pericentriolar material requires the fully assembled γ-TuRCs, and Dgp71WD could play an active role in this process. The absence of γ-tubulin recruitment along the spindle microtubules and at the midbody can also be explained by distinct properties of docking proteins at centrosomes and at noncentrosomal sites. Actually, novel proteins in charge of the recruitment of γ-tubulin complexes along spindle microtubules have recently been identified in fission yeast (Sawin et al., 2004; Janson et al., 2005).
Poles lacking complete γ-TuRC fulfill basic microtubule organization
In contrast with the impairment of microtubule assembly from the pericentriolar material after removal of γ-TuSC components (Barbosa et al., 2000; Raynaud-Messina et al., 2004; Colombie et al., 2006), the pericentriolar material appears to remain active as a microtubule-nucleating center after down-regulation of specific γ-TuRC proteins, as shown by the presence of astral microtubules identified both by immunofluorescence and electron microscopy (unpublished data). Moreover, microtubules containing 13 protofilaments were still nucleated, challenging the “template model” (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1; Moritz and Agard, 2001). This indicates that preassembly of cytosolic γ-TuRCs does not seem required for the formation of canonical microtubules. It is not excluded that a ringlike structure could be assembled from γ-TuSCs alone or in combination with small amounts of γ-TuRC–specific proteins at the pericentriolar level, although the purified γ-TuSC does not assemble into larger structures in vitro (Oegema et al., 1999). In that case, such ringlike complexes would exhibit stoichiometries and protein compositions different from γ-TuRCs.
Our observations could reconcile the findings of microtubule nucleation in animal cells and in S. cerevisiae. Moreover, after depletion of γ-TuRC–specific components, recruitment of γ-TuSC appeared sufficient, in most of the cases, to control the formation of spindles competent for chromosome segregation. However, mitotic processes were partly disrupted in cells lacking γ-TuRCs, leading to a transient prometaphase accumulation and a poor density of spindle microtubules. Several possibilities could account for these defects, such as a lower efficiency of γ-TuSCs compared with γ-TuRCs in microtubule nucleation (Oegema et al., 1999; Gunawardane et al., 2000), the presence of distinct binding sites for γ-TuSCs and γ-TuRCs, or the loss of γ-TuRC recruitment along spindle microtubules, which would affect the organization or the dynamics of specific microtubule arrays.
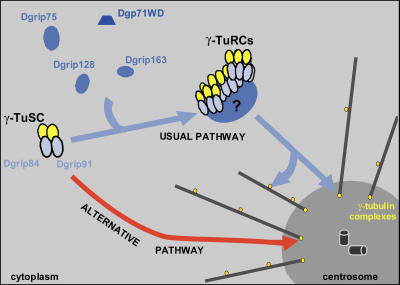
Collectively, our results strongly suggest that the assembly of γ-TuRCs is not essential for γ-tubulin–dependent microtubule nucleation at the centrosome, but instead is required for other, noncentrosomal localization of γ-tubulin. In D. melanogaster, γ-TuRCs may target a fully organized “nucleation machinery” to the sites of nucleation; γ-TuSC would act as a functional unit, whereas γ-TuRC–specific proteins would rather play a more refined role in regulating or optimizing of microtubule arrays during mitosis. γ-Tubulin can be targeted to the poles by the direct docking of γ-TuSCs that exert basic nucleation activity (Fig. 8). This γ-TuSC recruitment could be a “salvage pathway,” involved only when the dominant microtubule assembly mechanism mediated by γ-TuRCs is impaired or as a physiological pathway acting in parallel to γ-TuRC nucleation activity. Altogether, our data should prompt the reexamination of the current nucleation models.
Figure 8.
Model for γ-tubulin recruitment during mitosis in D. melanogaster. γ-Tubulin is targeted to the centrosome as γ-TuRC, but it appears that γ-tubulin complexes that are different from γ-TuRC, probably γ-TuSC, could be directly addressed to the poles.
Materials and methods
Cell culture and RNA-mediated interference
RNAi was performed in S2 D. melanogaster cells (Schneider, 1972) according to Clemens et al., (2000). Cells were treated twice with RNAi at days one and five and harvested on day seven for immunoblottting and immunofluorescence staining. To perform cosilencing, cells were incubated in the same way, with the four double-stranded RNAs (dsRNAs) against Dgrip75, Dgrip128, and Dgrip163, and Dgp71WD together (20 mM each). The dsRNAs used correspond to positions 717–1,577, relative to start of translation, for Dgrip75 (cDNA clone LD 19773); 157–918, relative to the start position for Dgrip128 (clone GH 21414); 141–846, relative to the start position for Dgrip163 (clone RE 43571); and 145–849, relative to the start position for Dgp71WD (clone RE59956). These dsRNAs were generated from the cDNA plasmid clones as described in Raynaud-Messina et al. (2004).
Fly strains
Strains y 1 w 118 or w 118 were used as control strains, and mutant strains Dgrip75 175 (Schnorrer et al., 2002), Dgrip163GE27087, and Dgp71WDGE30807 (GenExel, Inc.) were used for the study. Each mutant chromosome had been balanced over CyO, P[Kr:Gal4], P[UAS:GFP]or TM3,Sb,P[Kr:Gal4], P[UAS:GFP] (Casso et al., 2000). The GFP-balanced strains were used to select the homozygous mutant larvae. Viability of 100 selected larvae was studied during 28 d. The deficiencies Df(2L)Exel7071 and Df(3L)Exel6115 uncovering Dgp71WD or Dgrip163, respectively, were used to produce hemizygous flies. Note that Dgp71WD is referred as Dgrip71 in the Flybase database (http://flybase.bio.indiana.edu).
Antibodies
The mouse monoclonal antibodies T-5168, GTU88 (Sigma-Aldrich), and 1501 (CHEMICON International, Inc.) were used to stain α-tubulin, γ-tubulin, and actin, respectively. The following rabbit antibodies were raised: R62 specifically directed against D. melanogaster 23C γ-tubulin (Raynaud-Messina et al., 2004); R403 against the COOH-terminal peptide of Dgrip91 (-CRLDFNEYYKKRDTNLSK) for Western blot and R7075 against the recombinant COOH-terminal Dgrip91 (415–917 aa) for immunofluorescence (Colombie et al., 2006); and R367 against the recombinant full-length Dgrip84. R300 was raised against recombinant full-length Dgrip75 and affinity purified on recombinant Dgrip75, which is produced in Escherichia coli, R267 was raised against the recombinant COOH-terminal Dgp71WD (486–647 aa), and R360 against the recombinant COOH-terminal Dgrip163 (1150–1352 aa) was used for Western blot. For γ-tubulin detection, we used GTU88 for Western blot analysis and R62 for immunofluorescence experiments, except for double labeling, where monoclonal GTU88 antibody was used instead of polyclonal R62 antibody.
We also used Rb666 against BubR1 (Logarinho et al., 2004; gift from C. Sunkel, Universidad do Porto, Porto, Portugal), Rb3133 against Asp (Saunders et al., 1997; gift from D. Glover, University of Cambridge, Cambridge, UK), R19 against Cnn (Heuer et al., 1995; gift from T.C. Kaufman, Howard Hughes Institute, Indiana University, Bloomington, IN), and antibodies against Dgrip128 and Dgrip163 (Gunawardane et al., 2000; gift from Y. Zheng, Howard Hughes Institute, Carnegie Institution of Washington, Baltimore, MD).
Sucrose gradients
5–40% sucrose gradient was prepared as described previously (Oegema et al., 1999). Cell extracts (500 μg of protein) were overlaid onto the gradient and centrifuged for 4 h and 30 min at 4°C in a swinging rotor (SW55.1; Beckman Coulter) at 45,000 rpm (average 150,000 g). 10 0.5-ml fractions were collected and 250 μl of each fraction were precipitated with cold methanol (−20°C). The nine fractions corresponding to the soluble part of the extracts were analyzed by immunoblotting. The calibration of the gradient was determined by running in parallel 0–3-h D. melanogaster embryonic extracts that contain γ-TuSCs and γ-TuRCs.
Western blotting
Protein extracts from cultured cells (Raynaud-Messina et al., 2004) and from total larval brains (Colombie et al., 2006) were prepared and subjected to Western blot analyses (7.5% polyacrylamide gel and SDS [Sigma-Aldrich]). Apparent masses were determined by comparison with the SDS-PAGE molecular weight standards (broad range), which were obtained from Bio-Rad Laboratories.
Cytological analysis and microscopy techniques
For immunostaining, S2 cultured cells and semisquashed L3 larval brains were fixed and permeabilized as previously described (Colombie et al., 2006). DAPI staining of squashed third instar larvae was performed as described previously (Sunkel et al., 1995). For detection, secondary antibodies conjugated to Alexa Fluor 488 or 568 (Invitrogen) were used. Fluorescence microscopy was performed on a microscope (Axiovert; Carl Zeiss MicroImaging, Inc) equipped with a Z-motor, using 100×, 1.4 NA, objectives. Z-series images were acquired with a camera and software (Axiocam MRm and Axiovision; Carl Zeiss MicroImaging, Inc.). S2 cell or brain images were subsequently deconvolved using Axiovision, and Z-planes were projected onto a single view. The percentages of the different mitotic phenotypes were determined with confidence intervals calculated for a P95. Usually, image processing and quantification of fluorescence were done using Photoshop (Adobe). For quantification of polar γ-tubulin by immunofluorescence, we measured the maximal signal obtained after γ-tubulin staining. The signal of the camera was proportional to the fluorescence intensity, and the background and the maximal fluorescence values were adjusted to 0 and 25, respectively (Lajoie-Mazenc et al., 1994).
Online supplemental material
Fig. S1 shows colabeling of γ-tubulin with associated proteins in Dgrip75-depleted cells. Fig. S2 shows phenotypes observed after Dgp71WD depletion in S2 cells. Fig. S3 shows mitotic phenotypes in the Dgp71WD mutant. Fig. S4 shows the number of microtubule protofilaments in mitotic cells after Dgrip75 RNAi treatment. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200511071/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Y. Zheng for the gift of Dgrip128 and Dgrip163 antibodies, Dr. D. Glover for Asp antibodies, and Dr. T.C. Kaufman for Cnn antibodies. We would particularly like to thank A. Merdes and J-E. Gairin for their critical comments.
This work was supported by Association pour la Recherche sur le Cancer (ARC; grant 4720XP0230F), Fonds Européen de Développement Régional, Action Concertée Incitative (grant 04 5 566), Centre National de la Recherche Scientifique (CNRS), and Pierre Fabre funds. C. Verollet was supported by the CNRS and the Laboratoires Pierre Fabre, and N. Colombié was supported by the Ministère de la Recherche and the ARC.
Abbreviations used in this paper: Cnn, centrosomin; dsRNA, double-stranded RNA; γ-TuRC, γ-tubulin ring complex; γ-TuSC, γ-tubulin small complex; P95, probability of 95%; RNAi, RNA interference.
References
- Akashi, T., Y. Yoon, and B.R. Oakley. 1997. Characterization of gamma-tubulin complexes in Aspergillus nidulans and detection of putative gamma-tubulin interacting proteins. Cell Motil. Cytoskeleton. 37:149–158. [DOI] [PubMed] [Google Scholar]
- Barbosa, V., R.R. Yamamoto, D.S. Henderson, and D. Glover. 2000. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14:3126–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casso, D., F. Ramirez-Weber, and T.B. Kornberg. 2000. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech. Dev. 91:451–454. [DOI] [PubMed] [Google Scholar]
- Clemens, J.C., C.A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B.A. Hemmings, and J.E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA. 97:6499–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombie, N., C. Verollet, P. Sampaio, A. Moisand, C. Sunkel, H.M. Bourbon, M. Wright, and B. Raynaud-Messina. 2006. The Drosophila {gamma}-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol. Biol. Cell. 17:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr, N., J. Sillibourne, and M. Bornens. 2005. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118:1565–1575. [DOI] [PubMed] [Google Scholar]
- Fava, F., B. Raynaud Messina, J. Leung Tack, L. Mazzolini, M. Li, J.C. Guillemot, D. Cachot, Y. Tollon, P. Ferrara, and M. Wright. 1999. Human 76p: a new member of the γ-tubulin–associated protein family. J. Cell Biol. 147:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, A., L. Vardy, M.A. Garcia, and T. Toda. 2002. A fourth component of the fission yeast γ-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when γ-tubulin function is compromised. Mol. Biol. Cell. 13:2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler, S., G. Pereira, A. Spang, M. Knop, S. Soues, J. Kilmartin, and E. Schiebel. 1996. The spindle pole body component Spc98p interacts with the gamma-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J. 15:3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., O.C. Martin, K. Cao, L. Zhang, K. Dej, A. Iwamatu, and Y. Zheng. 2000. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 151:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., O.C. Martin, and Y. Zheng. 2003. Characterization of a new gammaTuRC subunit with WD repeats. Mol. Biol. Cell. 14:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., K. Oegema, M. Kirkham, P. Gonczy, B. Habermann, and A.A. Hyman. 2002. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren, L., M.-H. Remy, I. Bazin, I. Callebaut, M. Wright, and A. Merdes. 2006. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, J.G., K. Li, and T.C. Kaufman. 1995. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development. 121:3861–3876. [DOI] [PubMed] [Google Scholar]
- Janson, M.E., T.G. Setty, A. Paoletti, and P.T. Tran. 2005. Efficient formation of bipolar microtubule bundles requires microtubule-bound γ-tubulin complexes. J. Cell Biol. 169:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian, M., Y. Tollon, I. Lajoie-Mazenc, A. Moisand, H. Mazarguil, A. Puget, and M. Wright. 1993. Gamma-tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J. Cell Sci. 105:145–156. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, S., and Y. Zheng. 2004. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol. Biol. Cell. 15:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., and C.L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., and E. Schiebel. 1997. Spc98p and Spc97p of the yeast gamma-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16:6985–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., and E. Schiebel. 1998. Receptors determine the cellular localization of a gamma-tubulin complex and thereby the site of microtubule formation. EMBO J. 17:3952–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., G. Pereira, S. Geissler, K. Grein, and E. Schiebel. 1997. The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 16:1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie-Mazenc, I., Y. Tollon, C. Détraves, M. Julian, A. Moisand, C. Gueth-Hallonet, A. Debec, I. Salles-Passador, A. Puget, H. Mazarguil, et al. 1994. Recruitment of antigenic gamma-tubulin during mitosis in animal cells: presence of gamma-tubulin in the mitotic spindle. J. Cell Sci. 107:2825–2837. [DOI] [PubMed] [Google Scholar]
- Logarinho, E., H. Bousbaa, J.M. Dias, C. Lopes, I. Amorim, A. Antunes-Martins, and C.E. Sunkel. 2004. Different spindle checkpoint proteins monitor microtubule attachment and tension at kinetochores in Drosophila cells. J. Cell Sci. 117:1757–1771. [DOI] [PubMed] [Google Scholar]
- Megraw, T.L., K. Li, L.R. Kao, and T.C. Kaufman. 1999. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 126:2829–2839. [DOI] [PubMed] [Google Scholar]
- Moritz, M., and D.A. Agard. 2001. Gamma-tubulin complexes and microtubule nucleation. Curr. Opin. Struct. Biol. 11:174–181. [DOI] [PubMed] [Google Scholar]
- Murphy, S.M., L. Urbani, and T. Stearns. 1998. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components Spc97p and Spc98p. J. Cell Biol. 141:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., A.M. Preble, U.K. Patel, K.L. O'Connel, D.P. Dias, M. Moritz, D. Agard, J.T. Stults, and T. Stearns. 2001. GCP5 and GCP6: two new members of the human γ-tubulin complex. Mol. Biol. Cell. 12:3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T., D.B.N. Vinh, D.K. Crawford, and T.N. Davis. 1998. A genetic analysis of interactions with Spc110p reveals distinct functions of Spc97p and Spc98p, components of the yeast gamma-tubulin complex. Mol. Biol. Cell. 9:2201–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B.R., C.E. Oakley, Y. Yoon, and M.K. Jung. 1990. Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 61:1289–1301. [DOI] [PubMed] [Google Scholar]
- Oegema, K., C. Wiese, O.C. Martin, R.A. Milligan, E. Iwamatsu, T.J. Mitchison, and Y. Zheng. 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh, J.L., E. Nogales, B.R. Oakley, K. McDonald, A.L. Pidoux, and W.Z. Cande. 2000. A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell. 11:1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud-Messina, B., A. Debec, Y. Tollon, M. Garès, and M. Wright. 2001. Differential properties of the two Drosophila γ-tubulin isotypes. Eur. J. Cell Biol. 80:643–649. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina, B., L. Mazzolini, A. Moisand, A.M. Cirinesi, and M. Wright. 2004. Elongation of centriolar microtubule triplets contributes to the formation of the mitotic spindle in gamma-tubulin-depleted cells. J. Cell Sci. 117:5497–5507. [DOI] [PubMed] [Google Scholar]
- Saunders, R.D.C., M.C. Avides, T. Howard, C. Gonzalez, and D.M. Glover. 1997. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol. 137:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., P.C. Lourenco, and H.A. Snaith. 2004. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14:763–775. [DOI] [PubMed] [Google Scholar]
- Schneider, I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27:353–365. [PubMed] [Google Scholar]
- Schnorrer, F., S. Luschnig, I. Koch, and C. Nüsslein-Volhard. 2002. γ-Tubulin 37C and γ-tubulin ring complex protein 75 are essential for bicoid RNA localization during Drosophila oogenesis. Dev. Cell. 3:685–695. [DOI] [PubMed] [Google Scholar]
- Spang, A., S. Geissler, K. Grein, and E. Schiebel. 1996. γ-Tubulin–like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J. Cell Biol. 134:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns, T., and M. Kirschner. 1994. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell. 76:623–637. [DOI] [PubMed] [Google Scholar]
- Sunkel, C.E., R. Gomes, P. Sampaio, J. Perdigao, and C. Gonzalez. 1995. Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 14:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., A. Yamagiwa, T. Nishimura, H. Mukai, and Y. Ono. 2002. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell. 13:3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada, Y., Y. Uetake, and R. Kuriyama. 2003. Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J. Cell Biol. 162:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy, L., and T. Toda. 2000. The fission yeast γ-tubulin complex is required in G1 phase and is a component of the spindle assembly checkpoint. EMBO J. 19:6098–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatram, S., J.J. Tasto, A. Feoktistova, J.L. Jennings, A.J. Link, and K.L. Gould. 2004. Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol. Biol. Cell. 15:2287–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh, D.B.N., J.W. Kern, W.O. Hancock, J. Howard, and T.N. Davis. 2002. Reconstitution and characterization of budding yeast γ-tubulin complex. Mol. Biol. Cell. 13:1144–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield, J.G., S. Bonaccorsi, and M. Gatti. 2001. The Drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis. J. Cell Biol. 153:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., T.J. Keating, A. Wilde, G.G. Borisy, and Y. Zheng. 2000. The role of Xgrip210 in γ-tubulin ring complex assembly and centrosome recruitment. J. Cell Biol. 151:1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., M.L. Wong, B. Alberts, and T. Mitchison. 1995. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 378:578–583. [DOI] [PubMed] [Google Scholar]
- Zimmerman, W.C., J. Sillibourne, J. Rosa, and S.J. Doxsey. 2004. Mitosis-specific anchoring of γtubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 15:3642–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.