Abstract
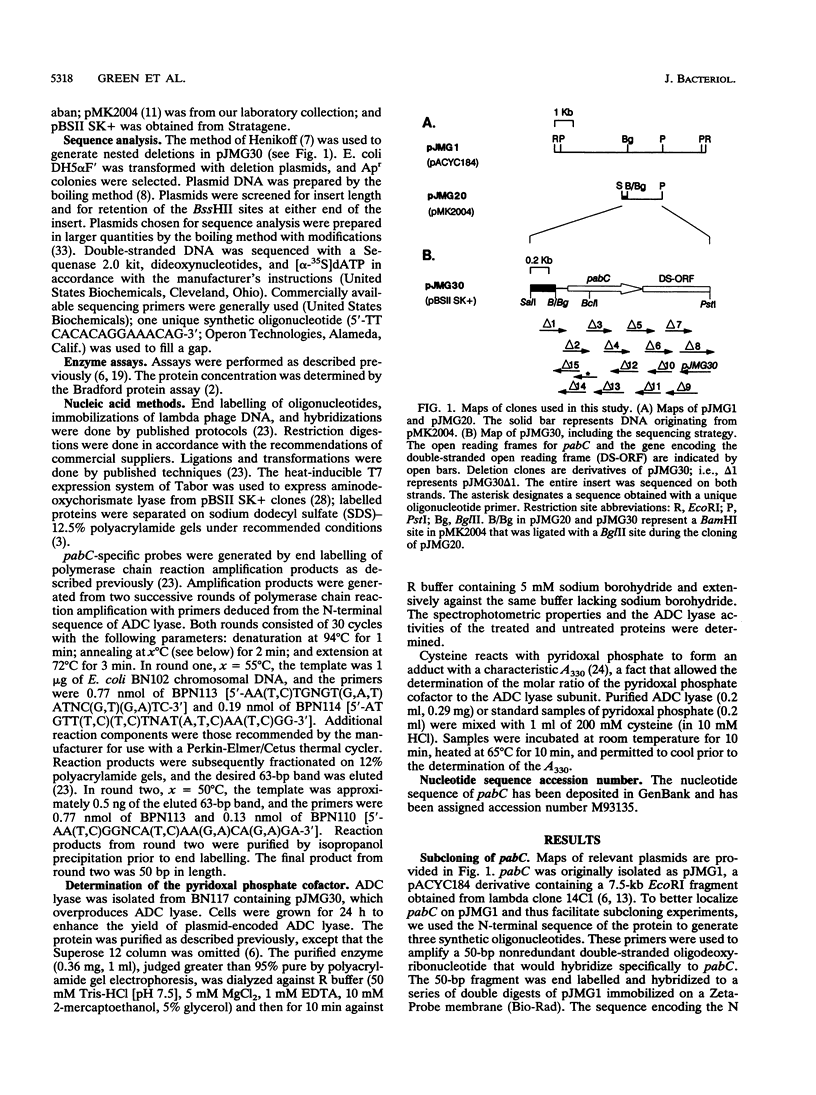
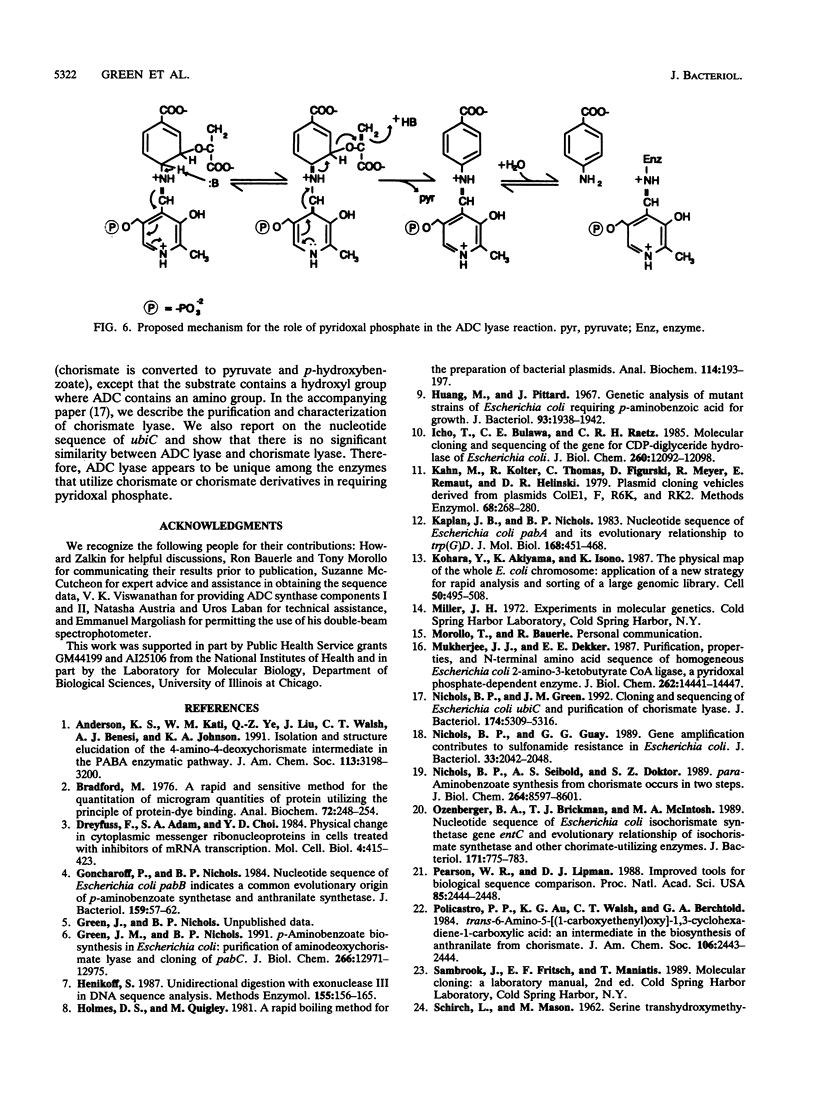
In Escherichia coli, p-aminobenzoate (PABA) is synthesized from chorismate and glutamine in two steps. Aminodeoxychorismate synthase components I and II, encoded by pabB and pabA, respectively, convert chorismate and glutamine to 4-amino-4-deoxychorismate (ADC) and glutamate, respectively. ADC lyase, encoded by pabC, converts ADC to PABA and pyruvate. We reported that pabC had been cloned and mapped to 25 min on the E. coli chromosome (J. M. Green and B. P. Nichols, J. Biol. Chem. 266:12971-12975, 1991). Here we report the nucleotide sequence of pabC, including a portion of a sequence of a downstream open reading frame that may be cotranscribed with pabC. A disruption of pabC was constructed and transferred to the chromosome, and the pabC mutant strain required PABA for growth. The deduced amino acid sequence of ADC lyase is similar to those of Bacillus subtilis PabC and a number of amino acid transaminases. Aminodeoxychorismate lyase purified from a strain harboring an overproducing plasmid was shown to contain pyridoxal phosphate as a cofactor. This finding explains the similarity to the transaminases, which also contain pyridoxal phosphate. Expression studies revealed the size of the pabC gene product to be approximately 30 kDa, in agreement with that predicted by the nucleotide sequence data and approximately half the native molecular mass, suggesting that the native enzyme is dimeric.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharoff P., Nichols B. P. Nucleotide sequence of Escherichia coli pabB indicates a common evolutionary origin of p-aminobenzoate synthetase and anthranilate synthetase. J Bacteriol. 1984 Jul;159(1):57–62. doi: 10.1128/jb.159.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. M., Nichols B. P. p-Aminobenzoate biosynthesis in Escherichia coli. Purification of aminodeoxychorismate lyase and cloning of pabC. J Biol Chem. 1991 Jul 15;266(20):12971–12975. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Huang M., Pittard J. Genetic analysis of mutant strains of Escherichia coli requiring p-aminobenzoic acid for growth. J Bacteriol. 1967 Jun;93(6):1938–1942. doi: 10.1128/jb.93.6.1938-1942.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icho T., Bulawa C. E., Raetz C. R. Molecular cloning and sequencing of the gene for CDP-diglyceride hydrolase of Escherichia coli. J Biol Chem. 1985 Oct 5;260(22):12092–12098. [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Nichols B. P. Nucleotide sequence of Escherichia coli pabA and its evolutionary relationship to trp(G)D. J Mol Biol. 1983 Aug 15;168(3):451–468. doi: 10.1016/s0022-2836(83)80295-2. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee J. J., Dekker E. E. Purification, properties, and N-terminal amino acid sequence of homogeneous Escherichia coli 2-amino-3-ketobutyrate CoA ligase, a pyridoxal phosphate-dependent enzyme. J Biol Chem. 1987 Oct 25;262(30):14441–14447. [PubMed] [Google Scholar]
- Nichols B. P., Green J. M. Cloning and sequencing of Escherichia coli ubiC and purification of chorismate lyase. J Bacteriol. 1992 Aug;174(16):5309–5316. doi: 10.1128/jb.174.16.5309-5316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Guay G. G. Gene amplification contributes to sulfonamide resistance in Escherichia coli. Antimicrob Agents Chemother. 1989 Dec;33(12):2042–2048. doi: 10.1128/aac.33.12.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Seibold A. M., Doktor S. Z. para-aminobenzoate synthesis from chorismate occurs in two steps. J Biol Chem. 1989 May 25;264(15):8597–8601. [PubMed] [Google Scholar]
- Ozenberger B. A., Brickman T. J., McIntosh M. A. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J Bacteriol. 1989 Feb;171(2):775–783. doi: 10.1128/jb.171.2.775-783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIRCH L. G., MASON M. Serine transhydroxymethylase. A study of the properties of a homogeneous enzyme preparation and of the nature of its interaction with substrates and pyridoxal 5-phosphate. J Biol Chem. 1963 Mar;238:1032–1037. [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slock J., Stahly D. P., Han C. Y., Six E. W., Crawford I. P. An apparent Bacillus subtilis folic acid biosynthetic operon containing pab, an amphibolic trpG gene, a third gene required for synthesis of para-aminobenzoic acid, and the dihydropteroate synthase gene. J Bacteriol. 1990 Dec;172(12):7211–7226. doi: 10.1128/jb.172.12.7211-7226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa K., Masu Y., Asano S., Tanaka H., Soda K. Thermostable D-amino acid aminotransferase from a thermophilic Bacillus species. Purification, characterization, and active site sequence determination. J Biol Chem. 1989 Feb 15;264(5):2445–2449. [PubMed] [Google Scholar]
- Wang L. M., Weber D. K., Johnson T., Sakaguchi A. Y. Supercoil sequencing using unpurified templates produced by rapid boiling. Biotechniques. 1988 Oct;6(9):839, 841-3. [PubMed] [Google Scholar]
- Ye Q. Z., Liu J., Walsh C. T. p-Aminobenzoate synthesis in Escherichia coli: purification and characterization of PabB as aminodeoxychorismate synthase and enzyme X as aminodeoxychorismate lyase. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9391–9395. doi: 10.1073/pnas.87.23.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]



