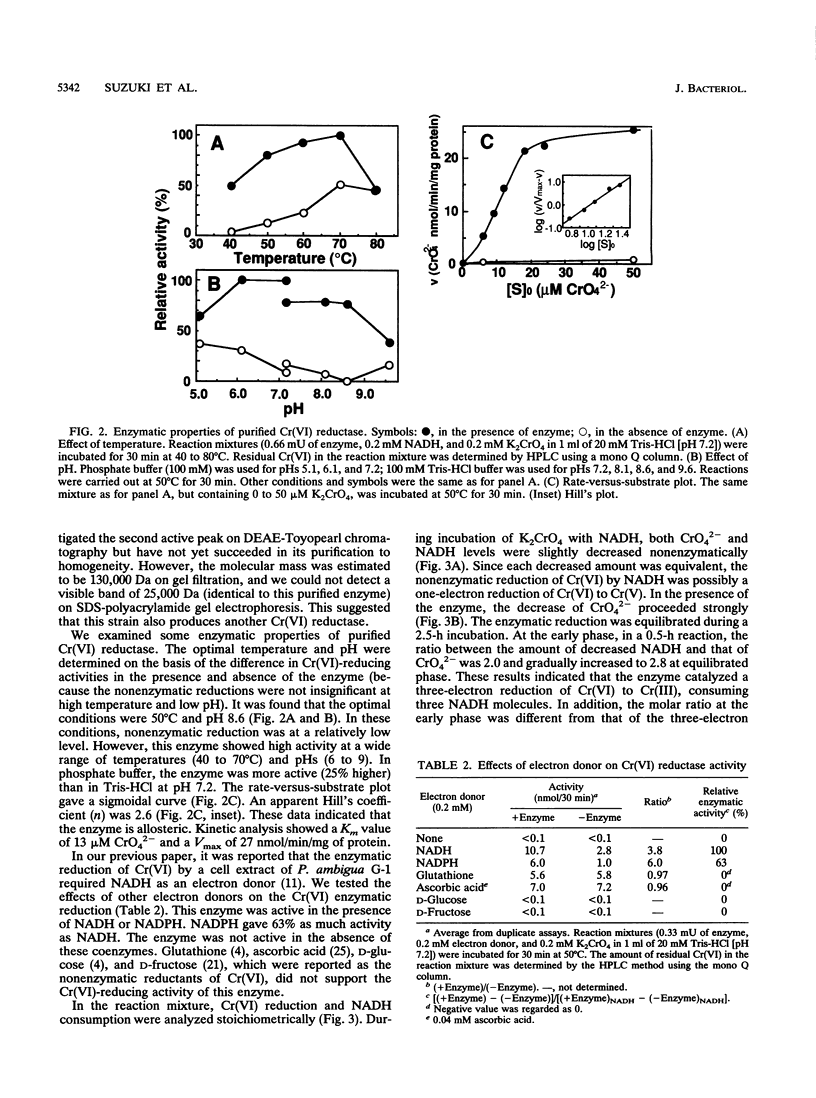
Abstract
An NAD(P)H-dependent Cr(VI) reductase (molecular weight = 65,000) was purified from a Cr(VI)-resistant bacterium, Pseudomonas ambigua G-1. Stoichiometric analysis of the enzymatic reaction showed that the enzyme catalyzed the reduction of 1 mol of Cr(VI) to Cr(III) while consuming 3 mol of NADH as an electron donor. Chromium(VI) was reduced to Cr(V) by one equivalent NADH molecule in the absence of the enzyme. Electron spin resonance analysis showed that Cr(V) species (g = 1.979) was formed during the enzymatic reduction. The amount of Cr(V) species formed was about 10 times larger than that of the nonezymatic reduction. These findings show that the Cr(VI) reductase reduced Cr(VI) to Cr(III) with at least two reaction steps via Cr(V) as an intermediate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Berkovits H. J., Floyd R. A., Wetterhahn K. E. Reaction of chromium(VI) with glutathione or with hydrogen peroxide: identification of reactive intermediates and their role in chromium(VI)-induced DNA damage. Environ Health Perspect. 1991 May;92:53–62. doi: 10.1289/ehp.919253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks R. B., Cooke R. T., Jr Chromate reduction by rabbit liver aldehyde oxidase. Biochem Biophys Res Commun. 1986 May 29;137(1):8–14. doi: 10.1016/0006-291x(86)91168-x. [DOI] [PubMed] [Google Scholar]
- Costa M. DNA-protein complexes induced by chromate and other carcinogens. Environ Health Perspect. 1991 May;92:45–52. doi: 10.1289/ehp.919245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Chandra A. L. Chromate reduction in Streptomyces. Experientia. 1990 Jul 15;46(7):731–733. doi: 10.1007/BF01939949. [DOI] [PubMed] [Google Scholar]
- De Flora S., Morelli A., Basso C., Romano M., Serra D., De Flora A. Prominent role of DT-diaphorase as a cellular mechanism reducing chromium(VI) and reverting its mutagenicity. Cancer Res. 1985 Jul;45(7):3188–3196. [PubMed] [Google Scholar]
- Fujii Y., Yoshioka T., Sasaki J. Fetal calf serum increased the zona pellucida penetrability of rat oocytes matured in vitro. Acta Med Okayama. 1990 Aug;44(4):203–208. doi: 10.18926/AMO/30425. [DOI] [PubMed] [Google Scholar]
- Garcia J. D., Jennette K. W. Electron-transport cytochrome P-450 system is involved in the microsomal metabolism of the carcinogen chromate. J Inorg Biochem. 1981 Jul;14(4):281–295. doi: 10.1016/s0162-0134(00)80286-x. [DOI] [PubMed] [Google Scholar]
- Goodgame D. M., Joy A. M. Relatively long-lived chromium(V) species are produced by the action of glutathione on carcinogenic chromium(VI). J Inorg Biochem. 1986 Mar;26(3):219–224. doi: 10.1016/0162-0134(86)80044-7. [DOI] [PubMed] [Google Scholar]
- Gruber J. E., Jennette K. W. Metabolism of the carcinogen chromate by rat liver microsomes. Biochem Biophys Res Commun. 1978 May 30;82(2):700–706. doi: 10.1016/0006-291x(78)90931-2. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Cervantes C., Silver S. Chromium reduction in Pseudomonas putida. Appl Environ Microbiol. 1990 Jul;56(7):2268–2270. doi: 10.1128/aem.56.7.2268-2270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- Kortenkamp A., Oetken G., Beyersmann D. The DNA cleavage induced by a chromium(V) complex and by chromate and glutathione is mediated by activated oxygen species. Mutat Res. 1990 Oct;232(2):155–161. doi: 10.1016/0027-5107(90)90120-s. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mikalsen A., Alexander J., Wallin H., Ingelman-Sundberg M., Andersen R. A. Reductive metabolism and protein binding of chromium(VI) by P450 protein enzymes. Carcinogenesis. 1991 May;12(5):825–831. doi: 10.1093/carcin/12.5.825. [DOI] [PubMed] [Google Scholar]
- Shi X. G., Dalal N. S. On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium(V) species. Arch Biochem Biophys. 1990 Mar;277(2):342–350. doi: 10.1016/0003-9861(90)90589-q. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. One-electron reduction of chromate by NADPH-dependent glutathione reductase. J Inorg Biochem. 1990 Sep;40(1):1–12. doi: 10.1016/0162-0134(90)80034-u. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Anion-exchange high-performance liquid chromatography of water-soluble chromium (VI) and chromium (III) complexes in biological materials. J Chromatogr. 1987 Apr 10;415(2):317–324. doi: 10.1016/s0378-4347(00)83223-5. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Application of fast protein liquid chromatography to the analysis of water-soluble chromium in nonbiological and biological substances. Ind Health. 1986;24(1):23–40. doi: 10.2486/indhealth.24.23. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch Toxicol. 1990;64(3):169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- Wang P. C., Mori T., Komori K., Sasatsu M., Toda K., Ohtake H. Isolation and Characterization of an Enterobacter cloacae Strain That Reduces Hexavalent Chromium under Anaerobic Conditions. Appl Environ Microbiol. 1989 Jul;55(7):1665–1669. doi: 10.1128/aem.55.7.1665-1669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. C., Mori T., Toda K., Ohtake H. Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol. 1990 Mar;172(3):1670–1672. doi: 10.1128/jb.172.3.1670-1672.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. C., Toda K., Ohtake H., Kusaka I., Yabe I. Membrane-bound respiratory system of Enterobacter cloacae strain HO1 grown anaerobically with chromate. FEMS Microbiol Lett. 1991 Feb;62(1):11–15. doi: 10.1016/0378-1097(91)90246-7. [DOI] [PubMed] [Google Scholar]


