Abstract
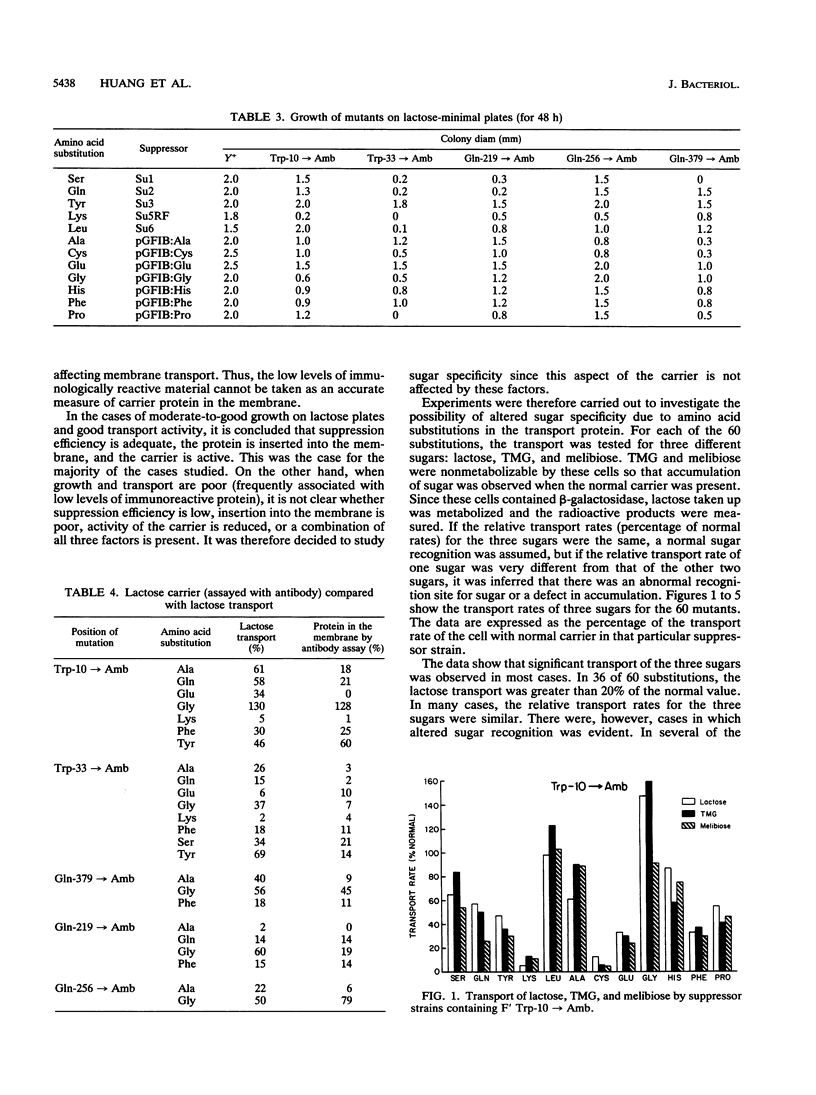
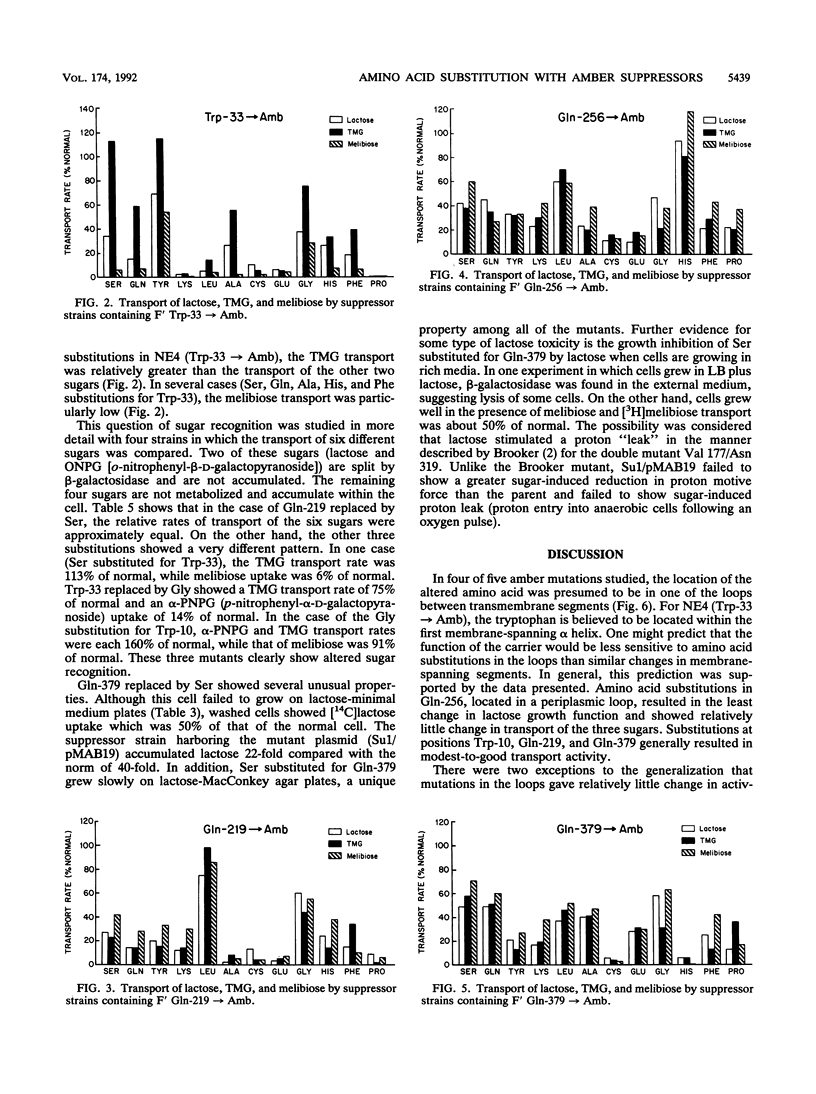
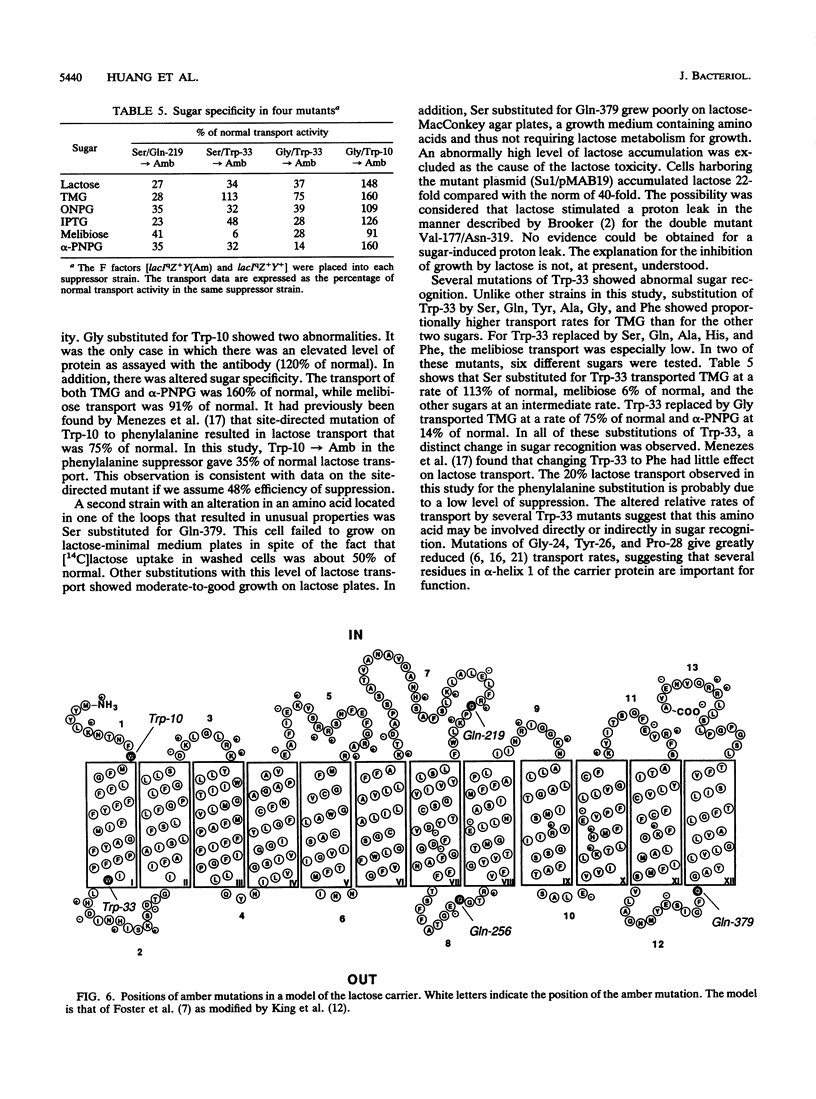
Five lacY mutants with amber stop codons at known positions were each placed into 12 different suppressor strains. The 60 amino acid substitutions obtained in this manner were tested for growth on lactose-minimal medium plates and for transport of lactose, melibiose, and thiomethylgalactoside. Most of the amino acid substitutions in the regions of the putative loops (between transmembrane alpha helices) resulted in a reasonable growth rate on lactose with moderate-to-good transport activity. In one strain (glycine substituted for Trp-10), abnormal sugar recognition was found. The substitution of proline for Trp-33 (in the region of the first alpha helix) showed no activity, while four additional substitutions (lysine, leucine, cysteine, and glutamic acid) showed low activity. Altered sugar specificity was observed when Trp-33 was replaced by serine, glutamine, tyrosine, alanine, histidine, or phenylalanine. It is concluded that Trp-33 may be involved directly or indirectly in sugar recognition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker R. J. An analysis of lactose permease "sugar specificity" mutations which also affect the coupling between proton and lactose transport. I. Val177 and Val177/Asn319 permeases facilitate proton uniport and sugar uniport. J Biol Chem. 1991 Mar 5;266(7):4131–4138. [PubMed] [Google Scholar]
- Brooker R. J. The lactose permease of Escherichia coli. Res Microbiol. 1990 Mar-Apr;141(3):309–315. doi: 10.1016/0923-2508(90)90004-a. [DOI] [PubMed] [Google Scholar]
- Brooker R. J., Wilson T. H. Isolation and nucleotide sequencing of lactose carrier mutants that transport maltose. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3959–3963. doi: 10.1073/pnas.82.12.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consler T. G., Tsolas O., Kaback H. R. Role of proline residues in the structure and function of a membrane transport protein. Biochemistry. 1991 Feb 5;30(5):1291–1298. doi: 10.1021/bi00219a019. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Herzlinger D., Carrasco N., Kaback H. R. Functional and immunochemical characterization of a mutant of Escherichia coli energy uncoupled for lactose transport. Biochemistry. 1985 Jan 1;24(1):221–229. doi: 10.1021/bi00322a032. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Hinkle P. V., Kaback H. R. Information content of amino acid residues in putative helix VIII of the lac permease from Escherichia coli. Biochemistry. 1990 Dec 11;29(49):10989–10994. doi: 10.1021/bi00501a017. [DOI] [PubMed] [Google Scholar]
- Hobson A. C., Gho D., Müller-Hill B. Isolation, genetic analysis, and characterization of Escherichia coli mutants with defects in the lacY gene. J Bacteriol. 1977 Sep;131(3):830–838. doi: 10.1128/jb.131.3.830-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Site-directed mutagenesis and ion-gradient driven active transport: on the path of the proton. Annu Rev Physiol. 1988;50:243–256. doi: 10.1146/annurev.ph.50.030188.001331. [DOI] [PubMed] [Google Scholar]
- King S. C., Hansen C. L., Wilson T. H. The interaction between aspartic acid 237 and lysine 358 in the lactose carrier of Escherichia coli. Biochim Biophys Acta. 1991 Feb 25;1062(2):177–186. doi: 10.1016/0005-2736(91)90390-t. [DOI] [PubMed] [Google Scholar]
- Kleina L. G., Masson J. M., Normanly J., Abelson J., Miller J. H. Construction of Escherichia coli amber suppressor tRNA genes. II. Synthesis of additional tRNA genes and improvement of suppressor efficiency. J Mol Biol. 1990 Jun 20;213(4):705–717. doi: 10.1016/S0022-2836(05)80257-8. [DOI] [PubMed] [Google Scholar]
- Kleina L. G., Miller J. H. Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol. 1990 Mar 20;212(2):295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., Püttner I. B., Kaback H. R. Site-directed mutagenesis of Pro327 in the lac permease of Escherichia coli. Biochemistry. 1988 Nov 1;27(22):8307–8310. doi: 10.1021/bi00422a003. [DOI] [PubMed] [Google Scholar]
- Markgraf M., Bocklage H., Müller-Hill B. A change of threonine 266 to isoleucine in the lac permease of Escherichia coli diminishes the transport of lactose and increases the transport of maltose. Mol Gen Genet. 1985;198(3):473–475. doi: 10.1007/BF00332941. [DOI] [PubMed] [Google Scholar]
- Menezes M. E., Roepe P. D., Kaback H. R. Design of a membrane transport protein for fluorescence spectroscopy. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1638–1642. doi: 10.1073/pnas.87.5.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Kleina L. G., Masson J. M., Abelson J., Miller J. H. Construction of Escherichia coli amber suppressor tRNA genes. III. Determination of tRNA specificity. J Mol Biol. 1990 Jun 20;213(4):719–726. doi: 10.1016/S0022-2836(05)80258-X. [DOI] [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Ogden R. C., Horvath S. J., Abelson J. Changing the identity of a transfer RNA. Nature. 1986 May 15;321(6067):213–219. doi: 10.1038/321213a0. [DOI] [PubMed] [Google Scholar]
- Overath P., Weigel U., Neuhaus J. M., Soppa J., Seckler R., Riede I., Bocklage H., Müller-Hill B., Aichele G., Wright J. K. Lactose permease of Escherichia coli: properties of mutants defective in substrate translocation. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5535–5539. doi: 10.1073/pnas.84.16.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepe P. D., Consler T. G., Menezes M. E., Kaback H. R. The lac permease of Escherichia coli: site-directed mutagenesis studies on the mechanism of beta-galactoside/H+ symport. Res Microbiol. 1990 Mar-Apr;141(3):290–308. doi: 10.1016/0923-2508(90)90003-9. [DOI] [PubMed] [Google Scholar]
- Rydén S. M., Isaksson L. A. A temperature-sensitive mutant of Escherichia coli that shows enhanced misreading of UAG/A and increased efficiency for some tRNA nonsense suppressors. Mol Gen Genet. 1984;193(1):38–45. doi: 10.1007/BF00327411. [DOI] [PubMed] [Google Scholar]


