Abstract
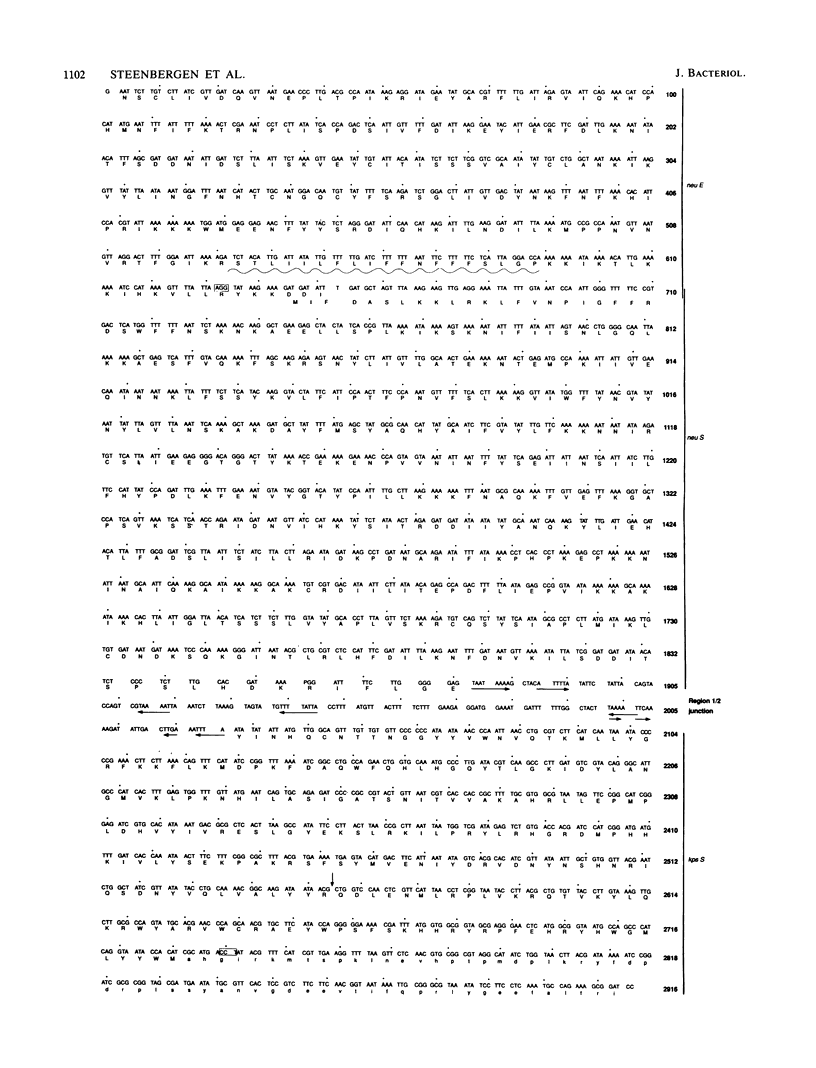
The polysialyltransferase (polyST) structural gene, neuS, for poly alpha 2,8sialic acid (PSA) capsule synthesis in Escherichia coli K1 was previously mapped near the kps region 1 and 2 junction (S. M. Steenbergen and E. R. Vimr, Mol. Microbiol. 4:603-611, 1990). Present Southern and colony blot hybridization results confirmed that neuS was a region 2 locus and indicated apparent homology with neuS from E. coli K92, bacteria that synthesize a sialyl alpha 2,8-2,9-linked polymer. A K1- mutant with an insertion mutation in neuS was complemented in trans by K92 neuS, providing direct evidence that neuS encoded the PSA polymerase. A 2.9-kb E. coli K1 kps subclone was sequenced to better characterize polyST. In addition to neuS, the results identified a new open reading frame, designated neuE, the linker sequence between regions 1 and 2, and the last gene of region 1, kpsS. The kpsS translational reading frame was confirmed by sequencing across the junction of a kpsS'-lacZ+ fusion. PolyST was identified by maxicell analysis of nested deletions and coupled in vitro transcription-translation assays. PolyST's derived primary structure predicted a 47,500-Da basic polypeptide without extensive similarity to other known proteins. PolyST activity was increased 31-fold and was membrane localized when neuS was cloned into an inducible expression vector, suggesting, together with the polyST primary structure, that polyST is a peripheral inner membrane glycosyltransferase. However, polyST could not initiate de novo PSA synthesis, indicating a functional requirement for other kps gene products. The existence of a sialyltransferase distinct from polyST was suggested by identification of a potential polyprenyl-binding motif in a C-terminal membrane-spanning domain of the predicted neuE gene product. Direct evidence for a quantitatively minor sialyltransferase activity, which could function to initiate PSA synthesis, was obtained by phenotypic analysis of mutants with multiple defects in sialic acid synthesis, degradation, and polymerization. The results provide an initial molecular description of K1 and K92 sialyltransferase complexes and suggest a possible common function for accessory kps gene products.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A., Sunshine J. L., Rutishauser U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol. 1991 Jul;114(1):143–153. doi: 10.1083/jcb.114.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright C. F., Orlean P., Robbins P. W. A 13-amino acid peptide in three yeast glycosyltransferases may be involved in dolichol recognition. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7366–7369. doi: 10.1073/pnas.86.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol Microbiol. 1989 Dec;3(12):1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Finke A., Jann B., Jann K. CMP-KDO-synthetase activity in Escherichia coli expressing capsular polysaccharides. FEMS Microbiol Lett. 1990 May;57(1-2):129–133. doi: 10.1016/0378-1097(90)90426-q. [DOI] [PubMed] [Google Scholar]
- Frosch M., Edwards U., Bousset K., Krausse B., Weisgerber C. Evidence for a common molecular origin of the capsule gene loci in gram-negative bacteria expressing group II capsular polysaccharides. Mol Microbiol. 1991 May;5(5):1251–1263. doi: 10.1111/j.1365-2958.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Robbins J. B., Liu T. Y., Gotschlich E. C., Orskov I., Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977 Jan;135(1):94–104. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Vimr E. R., Yu F., Bassler B., Troy F. A. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987 Mar 15;262(8):3553–3561. [PubMed] [Google Scholar]
- Hoyer L. L., Roggentin P., Schauer R., Vimr E. R. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl alpha 2----3 linkages. J Biochem. 1991 Sep;110(3):462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- Knudsen M. J., Troy F. A. Nuclear magnetic resonance studies of polyisoprenols in model membranes. Chem Phys Lipids. 1989 Nov;51(3-4):205–212. doi: 10.1016/0009-3084(89)90007-8. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J Bacteriol. 1985 Mar;161(3):861–867. doi: 10.1128/jb.161.3.861-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka M. S., Jr, Wright L. F., Silver R. P. Identification of two genes, kpsM and kpsT, in region 3 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1991 Aug;173(15):4603–4610. doi: 10.1128/jb.173.15.4603-4610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. N., Boulnois G. J., Roberts I. S. Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol. 1990 Nov;4(11):1863–1869. doi: 10.1111/j.1365-2958.1990.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen S. M., Vimr E. R. Mechanism of polysialic acid chain elongation in Escherichia coli K1. Mol Microbiol. 1990 Apr;4(4):603–611. doi: 10.1111/j.1365-2958.1990.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Valtersson C., van Duÿn G., Verkleij A. J., Chojnacki T., de Kruijff B., Dallner G. The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem. 1985 Mar 10;260(5):2742–2751. [PubMed] [Google Scholar]
- Vimr E. R., Aaronson W., Silver R. P. Genetic analysis of chromosomal mutations in the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1989 Feb;171(2):1106–1117. doi: 10.1128/jb.171.2.1106-1117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Lawrisuk L., Galen J., Kaper J. B. Cloning and expression of the Vibrio cholerae neuraminidase gene nanH in Escherichia coli. J Bacteriol. 1988 Apr;170(4):1495–1504. doi: 10.1128/jb.170.4.1495-1504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R. Map position and genomic organization of the kps cluster for polysialic acid synthesis in Escherichia coli K1. J Bacteriol. 1991 Feb;173(3):1335–1338. doi: 10.1128/jb.173.3.1335-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985 Nov;164(2):845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 1985 Nov;164(2):854–860. doi: 10.1128/jb.164.2.854-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber C., Troy F. A. Biosynthesis of the polysialic acid capsule in Escherichia coli K1. The endogenous acceptor of polysialic acid is a membrane protein of 20 kDa. J Biol Chem. 1990 Jan 25;265(3):1578–1587. [PubMed] [Google Scholar]
- Zapata G., Vann W. F., Aaronson W., Lewis M. S., Moos M. Sequence of the cloned Escherichia coli K1 CMP-N-acetylneuraminic acid synthetase gene. J Biol Chem. 1989 Sep 5;264(25):14769–14774. [PubMed] [Google Scholar]