Abstract
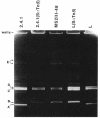
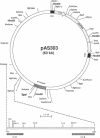
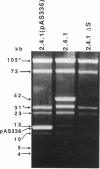
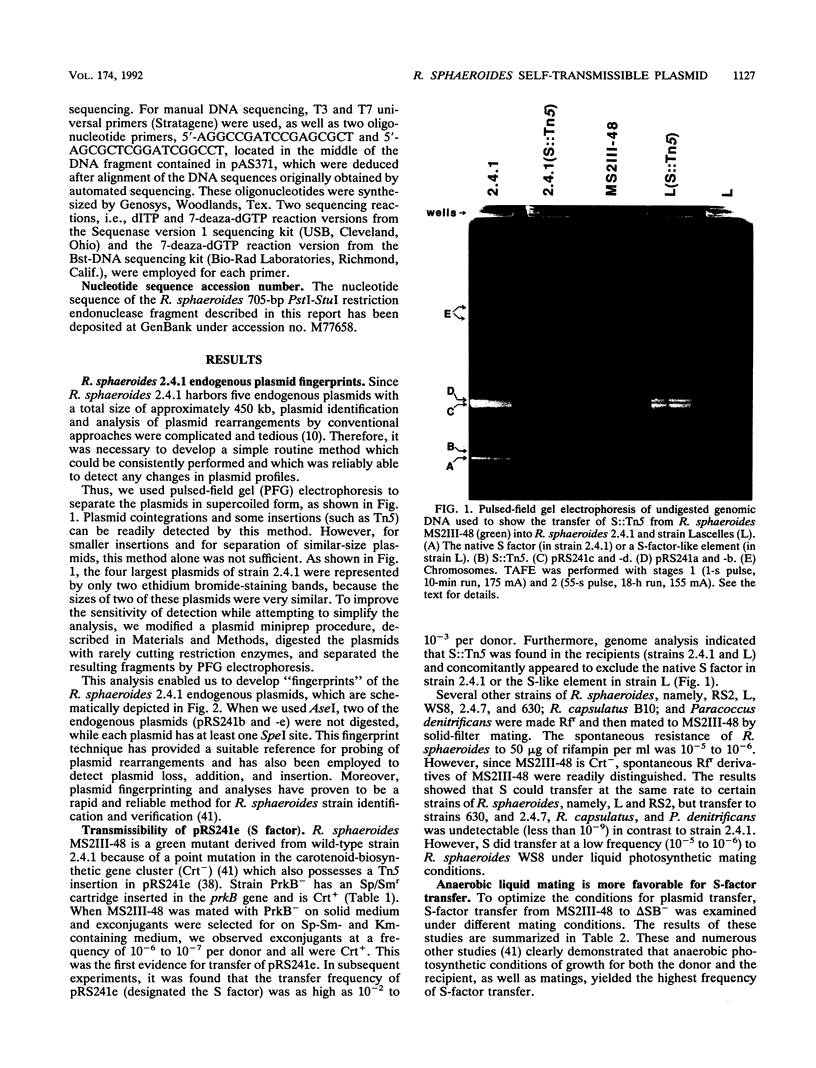
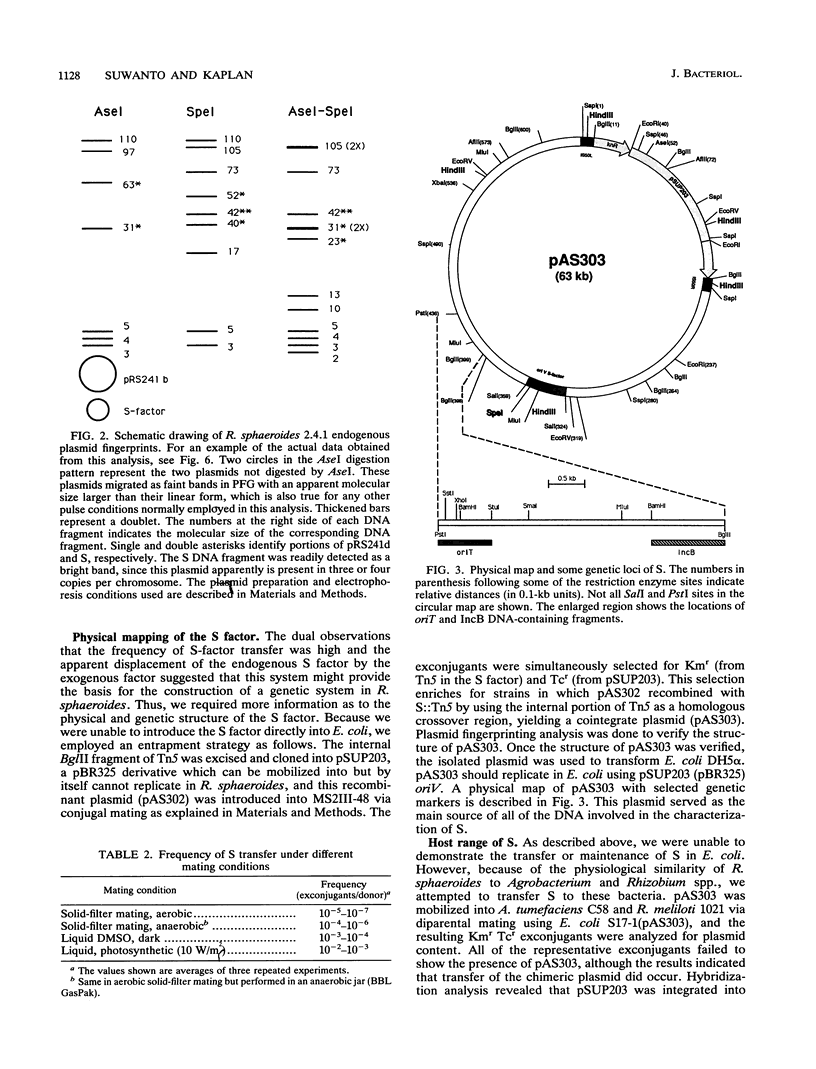
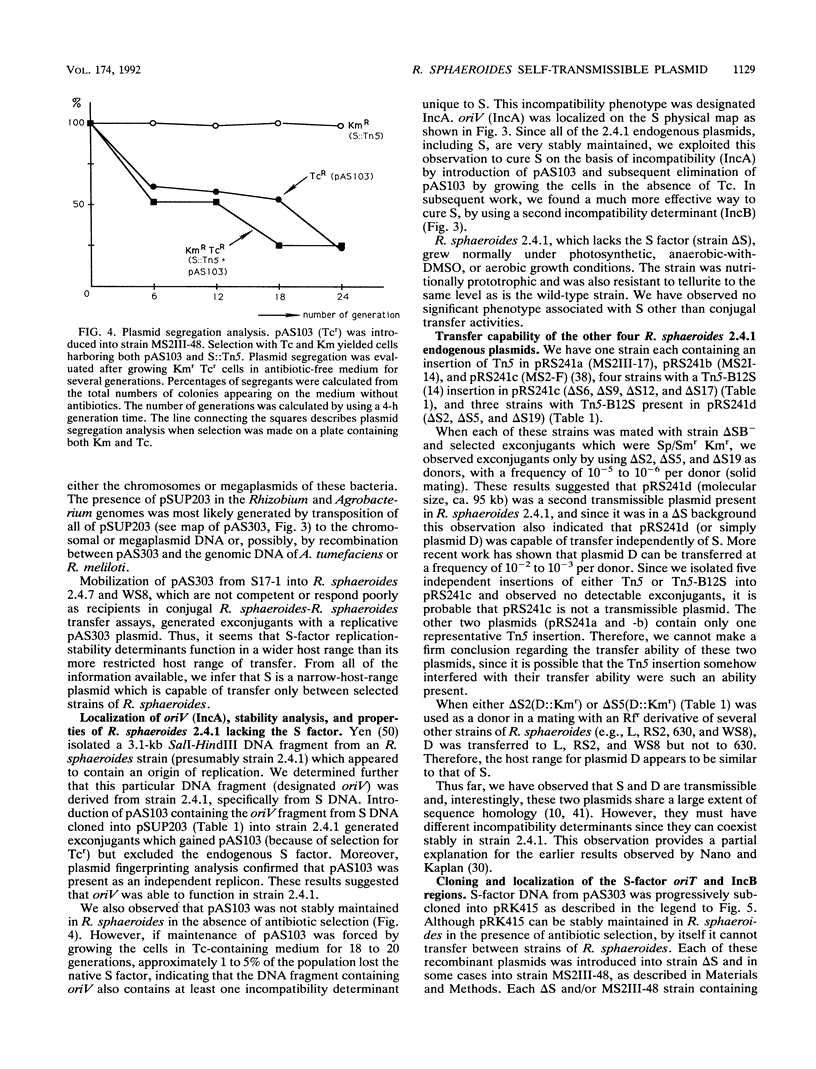
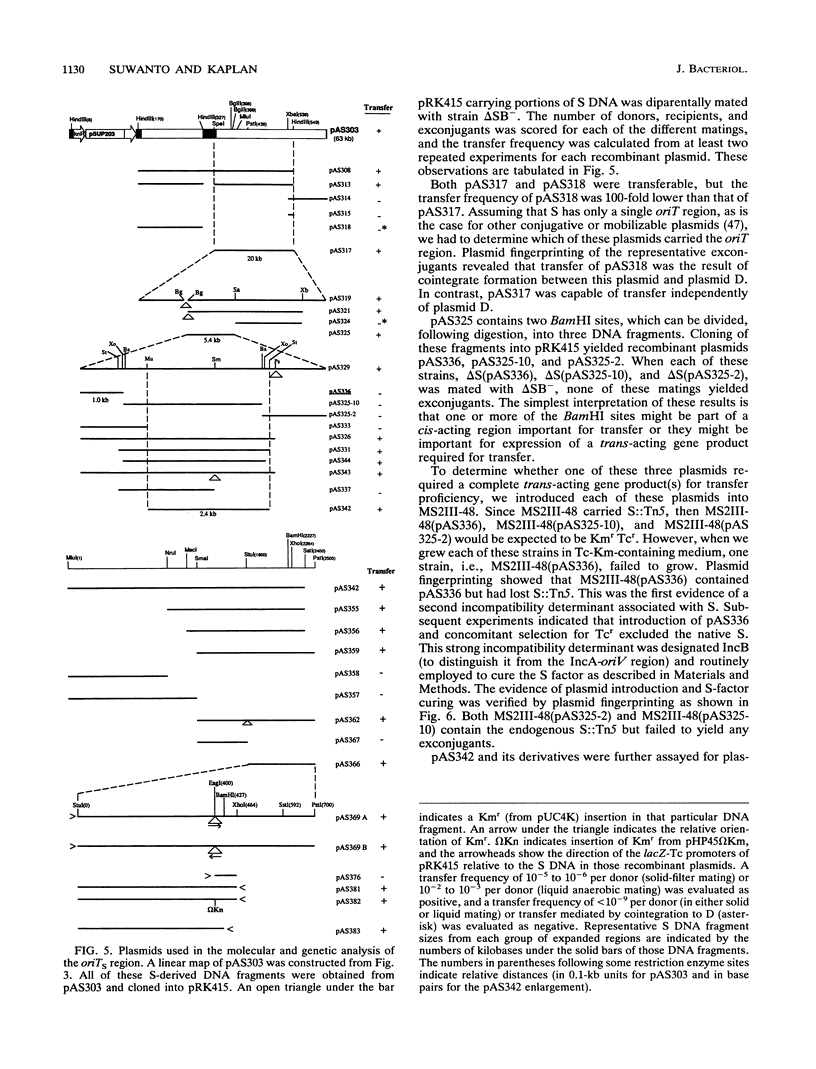
Rhodobacter sphaeroides 2.4.1 naturally harbors five cryptic endogenous plasmids (C. S. Fornari, M. Watkins, and S. Kaplan, Plasmid 11:39-47, 1984). The smallest plasmid (pRS241e), with a molecular size of 42 kb, was observed to be a self-transmissible plasmid which can transfer only to certain strains of R. sphaeroides. Transfer frequencies can be as high as 10(-2) to 10(-3) per donor under optimal mating conditions in liquid media in the absence of oxygen. pRS241e, designated the S factor, was also shown to possess a narrow host range, failing either to replicate or to be maintained in Escherichia coli, Agrobacterium tumefaciens, and Rhizobium meliloti. It was further revealed that one of the remaining four endogenous plasmids, pRS241d, was also transmissible at a frequency similar to that of the S. factor. As a cointegrate with pSUP203, S was maintained in E. coli, providing sufficient DNA from which a physical map of S could be constructed. Progressive subcloning of S-factor DNA, in conjunction with assays of plasmid transfer, led to the localization and identification of oriV (IncA), IncB, and the putative oriT locus. The DNA sequence of the 427 bp containing oriTs revealed topological similarity to other described oriT sequences, consisting of an A-T-rich DNA region, several direct and inverted repeats, and putative integration host factor (IHF)-binding sites, and was shown to be functional in promoting plasmid transfer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland S., Llosa M., Avila P., de la Cruz F. General organization of the conjugal transfer genes of the IncW plasmid R388 and interactions between R388 and IncN and IncP plasmids. J Bacteriol. 1990 Oct;172(10):5795–5802. doi: 10.1128/jb.172.10.5795-5802.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari C. S., Watkins M., Kaplan S. Plasmid distribution and analyses in Rhodopseudomonas sphaeroides. Plasmid. 1984 Jan;11(1):39–47. doi: 10.1016/0147-619x(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Tsai M. M., Luo Y. N., Deonier R. C. Deletion analysis of the F plasmid oriT locus. J Bacteriol. 1991 Feb;173(3):1012–1020. doi: 10.1128/jb.173.3.1012-1020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Kado C. I. Minimal region necessary for autonomous replication of pTAR. J Bacteriol. 1988 Jul;170(7):3170–3176. doi: 10.1128/jb.170.7.3170-3176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1749–1761. doi: 10.1128/jb.172.4.1749-1761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. F., Quandt J., O'Connell M. P., Pühler A. Direct selection for curing and deletion of Rhizobium plasmids using transposons carrying the Bacillus subtilis sacB gene. Gene. 1989 May 15;78(1):111–120. doi: 10.1016/0378-1119(89)90319-3. [DOI] [PubMed] [Google Scholar]
- Inamoto S., Yoshioka Y., Ohtsubo E. Site- and strand-specific nicking in vitro at oriT by the traY-traI endonuclease of plasmid R100. J Biol Chem. 1991 Jun 5;266(16):10086–10092. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya H., Yeates T. O., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: symmetry relations and sequence comparisons between different species. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9012–9016. doi: 10.1073/pnas.85.23.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Foster-Hartnett D. Transcriptional regulatory cascade of nitrogen-fixation genes in anoxygenic photosynthetic bacteria: oxygen- and nitrogen-responsive factors. Mol Microbiol. 1990 Nov;4(11):1793–1800. doi: 10.1111/j.1365-2958.1990.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Kües U., Looman A. C., Marquardt R., Stahl U. Determination and in vivo characterization of the basic replicon of natural plasmids of Methylomonas clara. Plasmid. 1989 Nov;22(3):224–235. doi: 10.1016/0147-619x(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Kües U., Stahl U. Replication of plasmids in gram-negative bacteria. Microbiol Rev. 1989 Dec;53(4):491–516. doi: 10.1128/mr.53.4.491-516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Meinhardt S. W., Kiley P. J., Kaplan S., Crofts A. R., Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985 Jan;236(1):130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J Bacteriol. 1989 Aug;171(8):4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E., Kaplan S. Plasmid rearrangements in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jun;158(3):1094–1103. doi: 10.1128/jb.158.3.1094-1103.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawsky A. R., Mills D. Identification and mapping of regions that confer plasmid functions and of sites for excisive recombination of plasmid pMMC7105. Mol Gen Genet. 1987 Feb;206(2):265–272. doi: 10.1007/BF00333583. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Sistrom W. R. Transfer of chromosomal genes mediated by plasmid r68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Aug;131(2):526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985 Jan;161(1):402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. W., Love J., Borghese R., Wall J. D. Identification and isolation of genes essential for H2 oxidation in Rhodobacter capsulatus. J Bacteriol. 1989 Feb;171(2):714–721. doi: 10.1128/jb.171.2.714-721.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]