Abstract
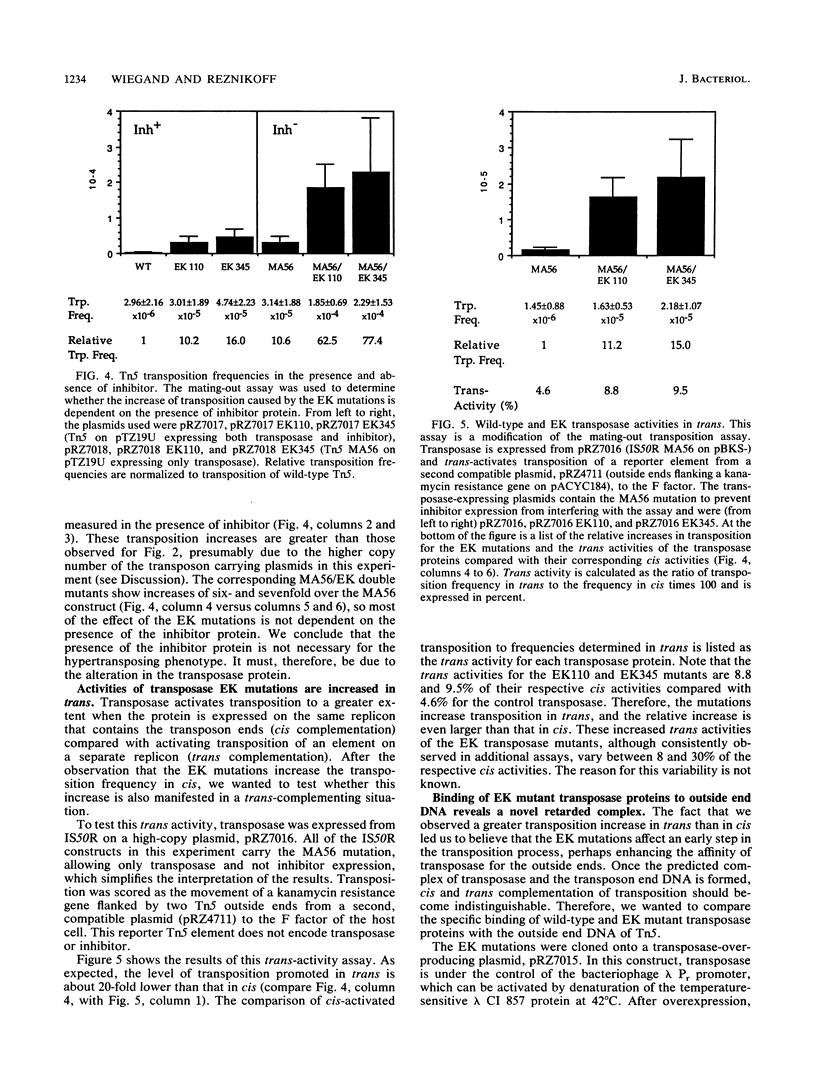
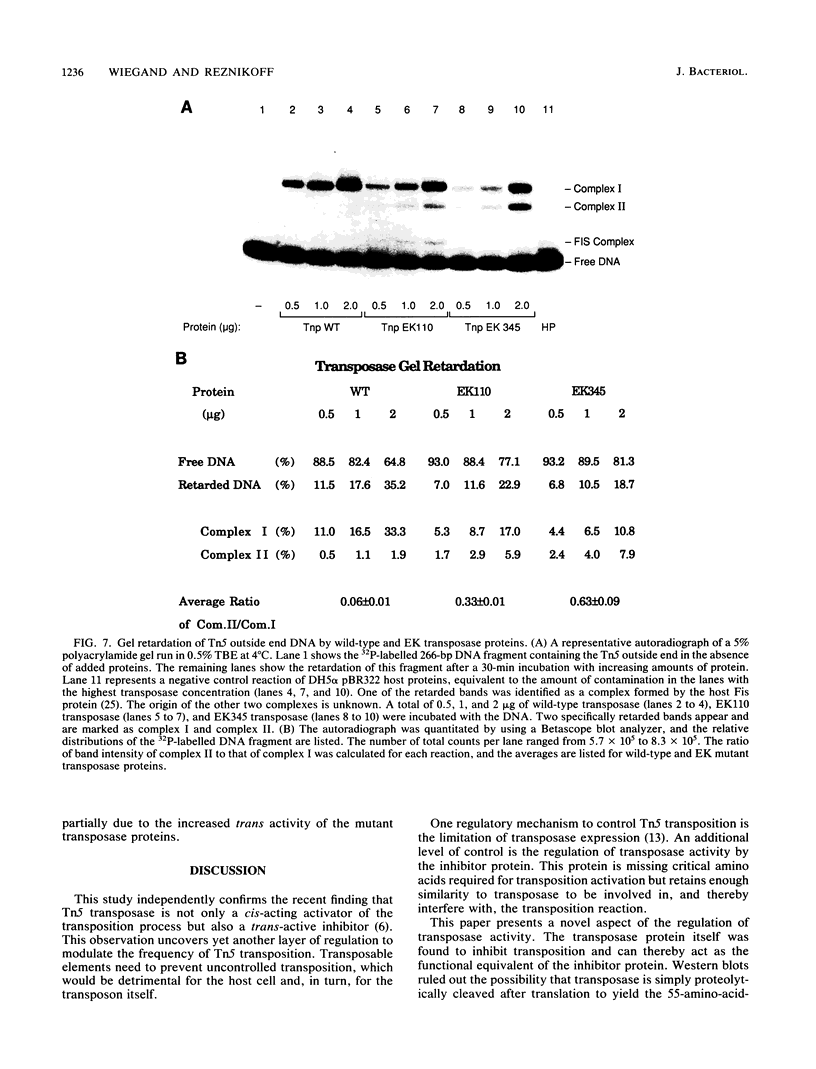
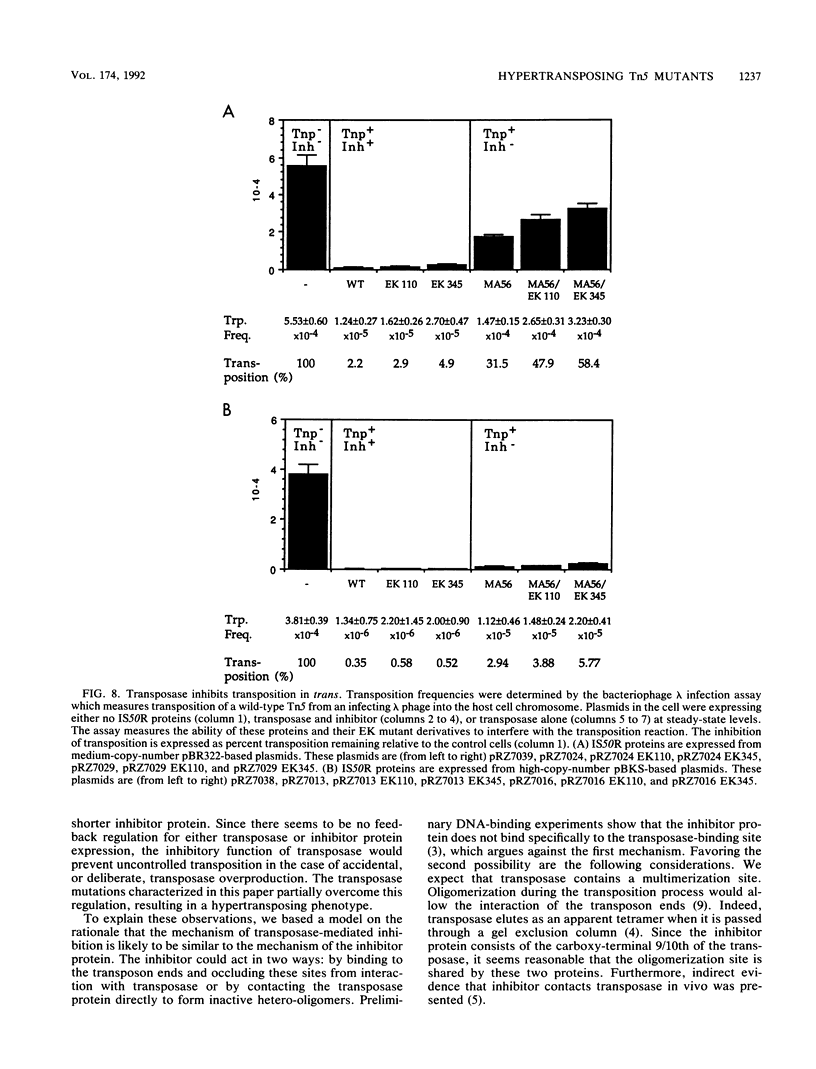
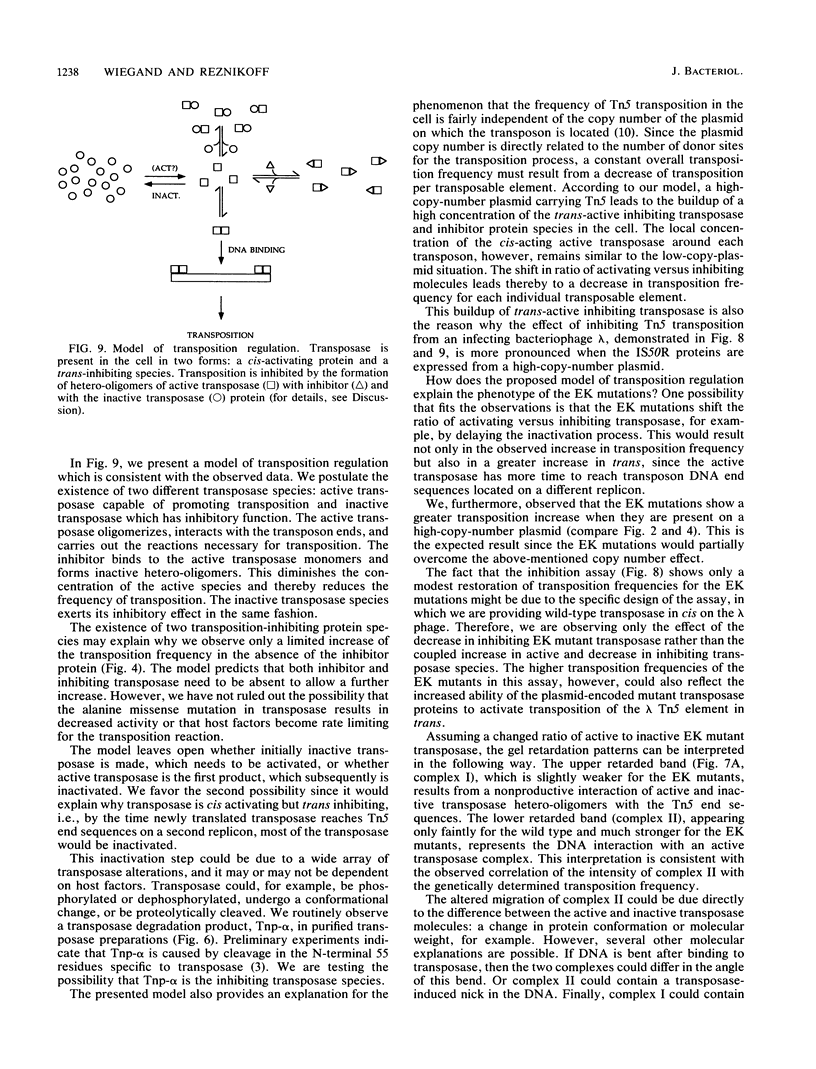
Transposition of Tn5 in Escherichia coli is regulated by two transposon-encoded proteins: transposase (Tnp), promoting transposition preferentially in cis, and the trans-acting inhibitor (Inh). Two separate transposase mutants were isolated that replace glutamate with lysine at position 110 (EK110) and at position 345 (EK345). The EK transposase proteins increase the Tn5 transposition frequency 6- to 16-fold in cis and enhance the ability of transposase to act in trans. The purified mutant transposase proteins interact with transposon outside end DNA differently from the wild-type protein, resulting in the formation of a novel complex in gel retardation assays. During characterization of the transposase proteins in the absence of inhibitor, we found that wild-type transposase itself has a transposition-inhibiting function and that this inhibition is reduced for the mutant proteins. We present a model for the regulation of Tn5 transposition, which proposes the existence of two transposase species, one cis-activating and the other trans-inhibiting. The phenotype of the EK transposase mutants can be explained by a shift in the ratio of these two species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DeLong A., Syvanen M. Membrane association of the Tnp and Inh proteins of IS50R. J Bacteriol. 1990 Sep;172(9):5516–5519. doi: 10.1128/jb.172.9.5516-5519.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong A., Syvanen M. Trans-acting transposase mutant from Tn5. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6072–6076. doi: 10.1073/pnas.88.14.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Syvanen M. DNA gyrase is a host factor required for transposition of Tn5. Cell. 1982 Aug;30(1):9–18. doi: 10.1016/0092-8674(82)90006-x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. Copy number control of Tn5 transposition. Genetics. 1984 May;107(1):9–18. doi: 10.1093/genetics/107.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. Role of the IS50 R proteins in the promotion and control of Tn5 transposition. J Mol Biol. 1984 Aug 25;177(4):645–661. doi: 10.1016/0022-2836(84)90042-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Yin J. C., Reznikoff W. S. Control of Tn5 transposition in Escherichia coli is mediated by protein from the right repeat. Cell. 1982 Oct;30(3):873–882. doi: 10.1016/0092-8674(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Reznikoff W. S. Transcriptional and translational initiation sites of IS50. Control of transposase and inhibitor expression. J Mol Biol. 1986 Dec 20;192(4):781–791. doi: 10.1016/0022-2836(86)90028-8. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Reznikoff W. S. Use of a Tn5 derivative that creates lacZ translational fusions to obtain a transposition mutant. Gene. 1988 Mar 31;63(2):277–285. doi: 10.1016/0378-1119(88)90531-8. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Makris J. C., Nordmann P. L., Reznikoff W. S. Integration host factor plays a role in IS50 and Tn5 transposition. J Bacteriol. 1990 Mar;172(3):1368–1373. doi: 10.1128/jb.172.3.1368-1373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris J. C., Nordmann P. L., Reznikoff W. S. Mutational analysis of insertion sequence 50 (IS50) and transposon 5 (Tn5) ends. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2224–2228. doi: 10.1073/pnas.85.7.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommas S. A., Syvanen M. Temporal control of transposition in Tn5. J Bacteriol. 1988 Feb;170(2):889–894. doi: 10.1128/jb.170.2.889-894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Uno Y., Yoshikawa M. The requirement for both DNA polymerase and 5' to 3' exonuclease activities of DNA polymerase I during Tn5 transposition. Mol Gen Genet. 1981;182(1):19–24. doi: 10.1007/BF00422761. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Uno Y., Yoshikawa M. lon-sulA regulatory function affects the efficiency of transposition of Tn5 from lambda b221 cI857 Pam Oam to the chromosome. Biochem Biophys Res Commun. 1987 Feb 13;142(3):879–884. doi: 10.1016/0006-291x(87)91495-1. [DOI] [PubMed] [Google Scholar]
- Schulz V. P., Reznikoff W. S. Translation initiation of IS50R read-through transcripts. J Mol Biol. 1991 Sep 5;221(1):65–80. doi: 10.1016/0022-2836(91)80205-9. [DOI] [PubMed] [Google Scholar]
- Syvanen M., Hopkins J. D., Clements M. A new class of mutants in DNA polymerase I that affects gene transposition. J Mol Biol. 1982 Jun 25;158(2):203–212. doi: 10.1016/0022-2836(82)90429-6. [DOI] [PubMed] [Google Scholar]
- Syvanen M. The evolutionary implications of mobile genetic elements. Annu Rev Genet. 1984;18:271–293. doi: 10.1146/annurev.ge.18.120184.001415. [DOI] [PubMed] [Google Scholar]
- Weinreich M. D., Makris J. C., Reznikoff W. S. Induction of the SOS response in Escherichia coli inhibits Tn5 and IS50 transposition. J Bacteriol. 1991 Nov;173(21):6910–6918. doi: 10.1128/jb.173.21.6910-6918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Wyman A. R., Botstein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49(2):253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- Yin J. C., Krebs M. P., Reznikoff W. S. Effect of dam methylation on Tn5 transposition. J Mol Biol. 1988 Jan 5;199(1):35–45. doi: 10.1016/0022-2836(88)90377-4. [DOI] [PubMed] [Google Scholar]
- Yin J. C., Reznikoff W. S. dnaA, an essential host gene, and Tn5 transposition. J Bacteriol. 1987 Oct;169(10):4637–4645. doi: 10.1128/jb.169.10.4637-4645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. C., Reznikoff W. S. p2 and inhibition of Tn5 transposition. J Bacteriol. 1988 Jul;170(7):3008–3015. doi: 10.1128/jb.170.7.3008-3015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]