Abstract
Aspergillus fumigatus infections cause high levels of morbidity and mortality in immunocompromised patients. Gliotoxin (GT), a secondary metabolite, is cytotoxic for mammalian cells, but the molecular basis and biological relevance of this toxicity remain speculative. We show that GT induces apoptotic cell death by activating the proapoptotic Bcl-2 family member Bak, but not Bax, to elicit the generation of reactive oxygen species, the mitochondrial release of apoptogenic factors, and caspase-3 activation. Activation of Bak by GT is direct, as GT triggers in vitro a dose-dependent release of cytochrome c from purified mitochondria isolated from wild-type and Bax- but not Bak-deficient cells. Resistance to A. fumigatus of mice lacking Bak compared to wild-type mice demonstrates the in vivo relevance of this GT-induced apoptotic pathway involving Bak and suggests a correlation between GT production and virulence. The elucidation of the molecular basis opens new strategies for the development of therapeutic regimens to combat A. fumigatus and related fungal infections.
Introduction
The saprophytic fungus Aspergillus fumigatus is a serious health hazard in hospitals (Latge, 1999, 2001). A. fumigatus is responsible for >90% of invasive aspergilloses (IA), with mostly fatal outcomes in immunocompromised patients suffering from AIDS, tuberculosis, cancer, or bone marrow/organ transplants (Latge, 1999; Bauters et al., 2005). The true incidence of A. fumigatus infections is underestimated because of the inherent difficulty of positive diagnosis (Latge, 1999).
Second-generation anti-fungals, such as amphotericin B lipid forms, echinocandins, and azoles, have only shown modest improvements in efficacy and at lower toxicity. Thus, anti– A. fumigatus therapy remains inadequate and, as a consequence, high morbidity and mortality from IA prevails (Latge, 1999; Bauters et al., 2005). One reason for this is our poor understanding of the pathobiology of A. fumigatus. A multitude of putative A. fumigatus virulence factors, such as extracellular metalloprotease, serine protease, aspartic protease, catalase, phospholipases, haemolysin, and the cytotoxin ASPF1 have been implicated in IA, but none has yet been shown to be involved in the pathogenesis of A. fumigatus in experimentally induced infections (Latge, 2001).
Gliotoxin (GT), an abundant mycotoxin produced by A. fumigatus and other fungi, such as Candida albicans, belongs to the epipolythiodioxopiperazine class of secondary metabolites (Taylor, 1971) and is characterized by a reactive disulfide bridge across the piperazine ring (Mullbacher et al., 1986). GT has been proposed to constitute a virulence factor in IA because of its immunosuppressive properties (Eichner and Mullbacher, 1984; Mullbacher and Eichner, 1984). GT has been shown in vitro to inhibit multiple processes associated with activation, differentiation, and/or effector functions of immune cells (Mullbacher et al., 1987; Waring et al., 1988a; Gardiner et al., 2005). This includes activation of NF-κB (Pahl et al., 1996), neutrophil and macrophage oxidative killing (Mullbacher et al., 1985; Murayama et al., 1996; Tsunawaki et al., 2004), polymorphonuclear neutrophils chemotaxis (Shah et al., 1998), polymorphonuclear neutrophils and macrophage phagocytosis (Mullbacher et al., 1985; Eichner et al., 1986; Murayama et al., 1996; Shah et al., 1998), activation of cytolytic T cells (Mullbacher and Eichner, 1984; Waring et al., 1988a), and IFNγ production by CD4+ lymphocytes (Wichmann et al., 2002). Most important, GT was found to induce mammalian cell apoptosis (Waring et al., 1988b; Sutton et al., 1994) accompanied by the production of reactive oxygen species (ROS) and mitochondrial membrane disruption (Eichner et al., 1988; Zhou et al., 2000; Suen et al., 2001; Kweon et al., 2003). The proposition that GT is a virulence factor is supported by more recent findings demonstrating that GT is expressed in vivo during experimental and human aspergillosis (Lewis et al., 2005) and that the decreased levels of pulmonary GT observed with an A. fumigatus mutant defective in LaeA, a global regulator of secondary metabolism, is associated with impaired virulence (Bok et al., 2005; unpublished data). However, it should be noted that LaeA regulates expression of a cassette of genes, including GT and other secondary metabolites. Thus, the virulence of the wild-type (wt) strain of A. fumigatus is not necessarily linked to GT alone.
Definitive evidence on the molecular basis of GT-mediated apoptosis and its relevance in the parasitized vertebrate host is still lacking. Many diverse stimuli, including irradiation, toxic drugs, and pathogens, transduce apoptotic signals to mammalian cells, resulting in the disruption of mitochondrial membranes (Kroemer and Reed, 2000; Dockrell, 2001; Green and Kroemer, 2004) and the subsequent release of apoptotic factors, such as cytochrome c and apoptosis-inducing factor (AIF; Liu et al., 1996; Susin et al., 1999; Pardo et al., 2001). Complex formation of cytochrome c with Apaf-1 and caspase-9 leads to further induction of effector caspases, i.e., caspase-3 and -7 (Li et al., 1997). Permeabilizing the mitochondrial membranes by diverse stimuli strictly depends on the proapoptotic Bcl-2 family members Bak and Bax (Marzo et al., 1998; Shimizu et al., 1999). This is indicated by the fact that deficiency in Bax and Bak renders cells resistant to numerous apoptotic stimuli (Wei et al., 2000, 2001). Although how Bax and Bak are regulated is still debated, it has become clear that they are somehow activated by BH3-only proteins, which trigger their conformational change, oligomerization, and pore forming activity (Letai et al., 2002; Willis et al., 2005). Among BH3-only molecules, Bid and Bim may directly activate Bax and Bak by a hit-and-run mechanism (Kuwana et al., 2005), whereas other members of this family seem to act by antagonizing the survival activity of antiapoptotic proteins, i.e., Bcl-2, Bcl-xL, and Mcl-1 (Huang and Strasser, 2000; Chen et al., 2005; Willis et al., 2005). However, it has not yet been studied by which mediators/effectors GT induces mitochondrial membrane disruption and apoptosis.
We show now for the first time that Bak, which constitutively resides on mitochondria (Griffiths et al., 1999), is the primary intracellular target in GT-mediated and ROS-facilitated apoptosis in vitro and that in a corticosteroid-based IA model, a knockout mouse strain lacking Bak is more resistant to A. fumigatus than wt mice.
Results
GT induces apoptosis in mouse embryonic fibroblast (MEF) cells
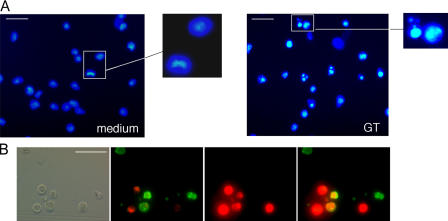
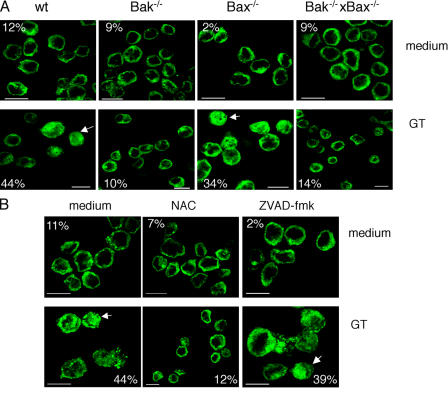
As GT may induce apoptosis and necrosis in mammalian cells, depending on the concentration of GT and the target cell used (Braithwaite et al., 1987; Hurne et al., 2002; Kweon et al., 2003; Orr et al., 2004), we first established optimal conditions that allowed us to readily monitor proapoptotic processes in MEFs. As shown in Fig. 1 A, 1 μM GT induced nuclear fragmentation in the majority of wt MEFs. Furthermore, ∼50% of these cells were apoptotic, i.e., had phosphatidylserine (PS) exposed (annexin V staining) without plasma membrane disruption, whereas the rest of the cells already showed secondary necrosis (loss of membrane integrity as shown by propidium iodide [PI] staining; Fig. 1 B; Pardo et al., 2004). Thus, 1 μM GT was used in all subsequent experiments.
Figure 1.
GT induces apoptosis in MEFs. wt MEFs were incubated with or without 1 μM GT for 4 h and stained with Hoechst 33342 (A) or annexin V–FITC plus PI (B), and fluorescence images were taken as described in Materials and methods. Bars: (A) 25 μm; (B) 40 μm.
GT-induced apoptosis depends on Bak but not Bax and/or Bid
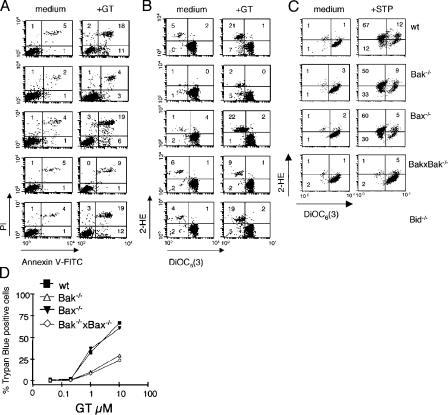
We next compared GT-induced PS exposure and PI staining between wt and knockout (−/−) MEFs. In addition, we studied the impact of GT on the Δψm and the production of ROS in these cells. As shown in Fig. 2 A, GT significantly increased the number of annexin V/PI–positive cells in wt, Bax−/−, and Bid−/− MEFs as compared with mock-treated cells, whereas Bak−/− and Bak−/− × Bax−/− MEFs did not. Similarly, GT treatment led to a significant reduction in the mitochondrial membrane potential (Δψm) and a parallel increase in ROS production in wt, Bax−/−, and Bid−/−, but not in Bak−/− and Bak−/− × Bax−/− MEFs (Fig. 2 B). It is our experience that there is considerable variation in the numbers of cells induced to express the respective proapoptotic markers from experiment to experiment. However, the differentials between experimental and control groups in individual experiments were always highly significant. This suggests that in MEFs, Bak, but not Bax, is critical for GT-induced loss of plasma membrane integrity and the Δψm. Moreover, Bid, which is known to activate Bak and Bax during apoptosis (Wei et al., 2001; Letai et al., 2002; Kuwana et al., 2005), seems to be dispensable for these processes. In support of a key role of Bak in GT-mediated cell death, we found that trypan blue exclusion was significantly reduced only in Bak−/− and Bak−/− × Bax−/−, but not in wt or Bax−/− MEFs (Fig. 2 D). In contrast to GT-induced apoptosis, the apoptosis-inducing drug staurosporine induced reduction in the Δψm and a parallel increase in ROS, which was only prevented in the absence of both Bak and Bax, as already described (Fig. 2 C; Wei et al., 2001).
Figure 2.
GT-induced apoptosis is Bak dependent. (A and B) wt, Bak−/−, Bax−/−, Bak−/− × Bax−/−, and Bid−/− MEFs were incubated with or without 1 μM GT for 4 h and analyzed by FACS for PS exposure (annexin V–FITC) and PI uptake (A) or Δψm loss (DiOC6[3]) and ROS generation (2-HE; B). (C) wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− MEFs were incubated with or without 1 μM staurosporine (STP) for 12 h and analyzed by FACS, as in B. The same cells were incubated with increasing amounts of GT for 4 h to determine the percentage of cell death (trypan blue exclusion) by microscopic inspection (D). Data shown in A, B, and D are representative of at least four independent experiments with similar outcome.
GT induces conformational change of Bak but not Bax, independent of Bid, caspase activation, or ROS generation
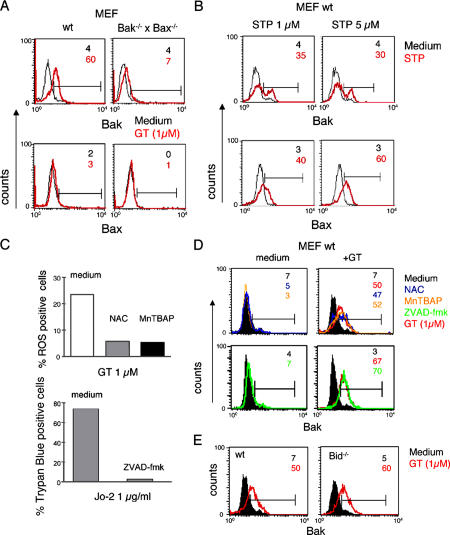
Upon their activation, Bak and Bax undergo conformational changes, leading to the exposure of their N-terminal domains (Hsu et al., 1997; Griffiths et al., 1999; Nechushtan et al., 1999). To test whether this process also occurs during GT treatment, wt and Bak−/− × Bax−/− MEFs were incubated with GT and subsequently analyzed by FACS with conformation-specific antibodies against the N termini of Bak or Bax. As shown in Fig. 3 A, GT was able to readily induce N-terminal epitope exposure in Bak but not in Bax. As expected, no (Bax) or only marginal staining (Bak) was seen with these antibodies in GT-activated Bak−/− × Bax−/− MEFs. There was no inherent failure of Bax to undergo an N-terminal conformational change in MEFs, as their treatment with staurosporine led to the expected N-terminal opening of Bax (Fig. 3 B; Griffiths et al., 1999; Wei et al., 2001).
Figure 3.
GT induces conformational change of Bak. (A and B) GT induces conformational change of Bak, but not Bax, in MEFs. wt and Bak−/− × Bax−/− MEFs were incubated for 4 h without (black) or with (red) either 1 μM GT (A) or 1 and 5 μM staurosporine (B) and analyzed by FACS for conformational changes of Bak and Bax using mAbs specific for the functionally active N-terminal region in each protein. Numbers given are the percentages of cells positive for active Bak or Bax (indicated by the horizontal bars). (C–E) GT-induced conformational change of Bak is independent of caspase activation, ROS generation, and the presence of Bid. (C) The ROS scavengers NAC and MnTBAP were tested for their efficiency in inhibiting ROS production by FACS analysis (2-HE) of wt MEFs treated with 1 μM GT for 4 h. To test for the caspase-inhibiting potency of ZVAD-fmk, MBL-2–Fas cells were treated with 1 μg/ml of the α-Fas antibody Jo-2 in the absence and presence of 100 μM ZVAD-fmk, and cell death was monitored by trypan blue exclusion. (D) wt MEFs were incubated with (red) or without (black) 1 μM GT for 4 h in the presence or absence of 100 μM ZVAD-fmk (green), 15 mM NAC, or 200 μM MnTBAP. The cells were analyzed by FACS for conformational changes of Bak as under A. (E) GT-induced conformational change of Bak is independent of Bid. wt and Bid−/− MEFs were treated with GT and FACS analyzed for conformational changes of Bak as in A. Numbers given are the percentages of cells positive for active Bak (indicated by the horizontal bars). Data shown are representative of at least three independent experiments with similar outcome.
To determine the order of events during Bak activation, ROS production, and caspase activation, we incubated GT-treated MEFs with the antioxidants N-acetylcysteine (NAC) or the manganese porphyrin Mn(III) tetrakis(4-benzoic acid) porphyrin chloride (MnTBAP; Faulkner et al., 1994; Day et al., 1997) or the pan-caspase inhibitor ZVAD-fmk (Pardo et al., 2004). NAC and MnTBAP were both effective as antioxidants, as they significantly reduced GT-induced ROS production in MEFs (Fig. 3 C). Moreover, ZVAD-fmk blocked apoptosis induced by the anti(α)-Fas mAb Jo-2 in MBL-2–Fas cells (Fig. 3 C). GT-induced N-terminal opening of Bak was unaffected by any of the three inhibitors (Fig. 3 D), indicating that ROS production and caspase activation occur downstream of Bak activation. Furthermore, as similar activation of Bak was seen in GT-treated wt and Bid−/− MEFs, GT-mediated conformational change of Bak is also independent of Bid (Fig. 3 E).
GT-induced mitochondrial and membrane damage depends on ROS production
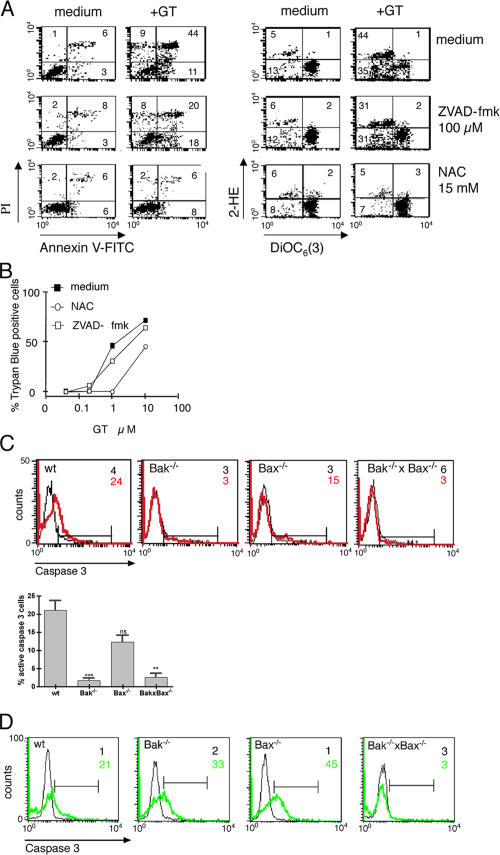
Although the association of ROS generation with GT- or CTL-mediated apoptosis is well documented (Kweon et al., 2003; Pardo et al., 2004), the contribution of ROS to cell death is still controversial (Chandra et al., 2000; Danial and Korsmeyer, 2004). We therefore tested the effect of NAC and MnTBAP on GT-induced plasma and mitochondrial membrane integrity and cell death. As shown in Fig. 4 A, the addition of NAC to wt MEFs at a concentration known to inhibit ROS generation (Fig. 3 C; Pardo et al., 2004) abrogated GT-induced PS exposure and plasma membrane permeability, as well as the reduction of the mitochondrial Δψm. Moreover, at 1 μM GT, NAC totally inhibited cell death (according to the absence of trypan blue staining; Fig. 4 B). Similar results were obtained with MnTBAP, indicating that the effect of NAC was due to its antioxidant properties (unpublished data). These data reveal that ROS generation is crucial for GT-induced changes of plasma membrane permeability, the mitochondrial Δψm, and cell death.
Figure 4.
GT-induced mitochondrial membrane perturbation and apoptosis are dependent on ROS generation, whereas only the latter partially depends on caspases. (A) wt MEFs were incubated with or without 1 μM GT for 4 h in the presence or absence of 100 μM of the pan-caspase inhibitor ZVAD-fmk (green) or 15 mM of the ROS scavenger NAC and analyzed by FACS for PS exposure (annexin V–FITC)/PI uptake and Δψm loss (DiOC6[3])/ROS generation (2-HE). (B) wt MEFs were incubated with increasing amounts of GT for 4 h in the presence or absence of ZVAD-fmk or NAC, and the percentage of cell death (trypan blue exclusion) was determined by microscopic inspection. (C) wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− MEFs were incubated with (red) or without (black) 1 μM GT for 4 h and analyzed by FACS for the activation of caspase-3, using an α–caspase-3 mAb (FITC labeled) against the active form of the enzyme. A representative FACS analysis represented as histogram (C, bottom) and a graph showing the mean ± SEM from four independent experiments (right) is shown. ns, P = 0.1730; **, P = 0.0002; ***, P = 0,0012. Analyzed by two-tailed unimpaired t test comparing wt versus knockouts. (D) wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− MEFs were incubated with (green) or without (black) 1 μM staurosporine for 4 h and analyzed by FACS for the activation of caspase-3, as in C.
GT-induced caspase-3 activation depends on Bak activation
To determine the role of caspases in the GT-induced reduction of the mitochondrial Δψm and apoptosis, we tested whether GT could induce cell death and changes in mitochondrial Δψm in the presence of the broad spectrum caspase inhibitor ZVAD-fmk. 100 μM ZVAD-fmk did not prevent loss of the mitochondrial Δψm in GT-treated cells, indicating that caspase activation was not needed for this event. However, ZVAD-fmk partially reduced GT-induced annexin V/PI staining (Fig. 4 A) but did not affect cell death (Fig. 4 B). These data also exclude a possible role of death receptor–mediated cell death, which is completely blocked by caspase inhibitors (Fig. 3 C; Longthorne and Williams, 1997). To test whether the major downstream effector caspase, caspase-3, was involved in these processes, and whether activation of this caspase was dependent on Bak, we performed a FACS analysis of wt and −/− MEFs using an α–caspase-3 mAb specific for the processed active form of caspase-3. As shown in Fig. 4 C, caspase-3 was significantly activated in GT-treated wt and Bax−/− but not in Bak−/− and Bak−/− × Bax−/− MEFs. In contrast, staurosporine induced caspase-3 activation in Bak−/− and Bax−/− but not in Bak−/− × Bax−/− MEFs (Fig. 4 D), again showing that both Bak and Bax can undergo conformational activation upon appropriate stimulus (Wei et al., 2001).
GT-induced release of cytochrome c depends on Bak activation and ROS production
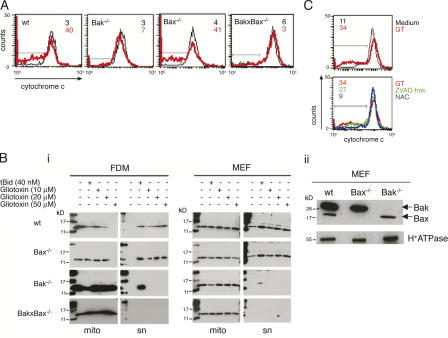
The activation of Bak and the subsequent production of ROS and the reduction of mitochondrial Δψm in response to GT indicate increased mitochondrial membrane permeability, leading to the release of apoptogenic factors such as cytochrome c and AIF. Although cytochrome c activates caspase-3 via the apoptosome (Li et al., 1997), AIF translocates to the nucleus and contributes to DNA fragmentation in a caspase-independent manner (Susin et al., 1999; Pardo et al., 2001). The release of both cytochrome c and AIF from mitochondria is absolutely dependent on activation of Bax, Bak, or both (Lindsten et al., 2000; Wei et al., 2001). To test whether GT-induced cytochrome c release selectively required Bak, wt, Bak−/−, Bax−/−, or Bak−/− × Bax−/− MEFs were incubated with GT and mitochondrial cytochrome c was quantitatively measured by FACS analysis (Fig. 5 A). In addition, AIF release was monitored by α-AIF immunofluorescence (Fig. 6 A). As shown in Fig. 5 A, mitochondrial cytochrome c was reduced in GT-treated wt and Bax−/− MEFs but retained in Bak−/− or Bak−/− × Bax−/− MEFs. In addition, a high portion of GT-treated wt and Bax−/− MEFs displayed cytosolic localization and nuclear translocation of AIF, whereas Bak−/− and Bak−/− × Bax−/− MEFs retained most of the AIF in the mitochondria (Fig. 6 A). These data confirm that GT induced mitochondrial membrane permeability; i.e., cytochrome c and AIF release occurs by a process selectively involving Bak. To test whether GT could directly act on mitochondrial Bak without the requirement of any cytosolic factors, such as, for example, a particular BH3-only protein, we compared GT-induced cytochrome c release on isolated mitochondria from wt MEFs with those of knockout MEFs and factor-dependent myeloids (FDMs). As shown in Fig. 5 B i, 10–50 μM GT caused cytochrome c release from isolated wt mitochondria in a dose-dependent manner. The requirement for much higher concentrations of GT to induce cytochrome c release in isolated mitochondria as compared with intact cells is in line with a previous study in which GT-mediated calcium release was analyzed (Schweizer and Richter, 1994). In fact, it is known that levels of GT determined intracellularly do exceed those originally applied in solution by up to 1,500-fold (Waring et al., 1994; Bernardo et al., 2003). The release of cytochrome c was as efficient as that induced by recombinant tBid, a known inducer of mitochondrial membrane permeability via Bak/Bax (Wei et al., 2000, 2001) and significantly greater than the background release of cytochrome c observed in mock-treated mitochondrial preparations. Strikingly, although mitochondria from Bax−/− showed similar GT-induced cytochrome c release as wt mitochondria, mitochondria from Bak−/− or Bak−/− × Bax−/− did not, irrespective of whether they were derived from MEFs or FDMs. This suggests that GT does not need cytosolic factors such as tBid or caspases to elicit cytochrome c release but may act directly on mitochondrial Bak or some as-yet-unknown mitochondrial protein that activates Bak with a potency similar to tBid. We excluded the possibility that the resistance of GT-facilitated cytochrome c released could be due to the absence of Bax on mitochondria, as appreciable amounts of this protein were detected on isolated, washed mitochondria from both wt and Bak−/− FDMs (Fig. 5 B ii). Moreover, tBid was capable of inducing cytochrome c release from mitochondria isolated from both Bak−/− FDMs and MEFs. This would not have been possible if they had no Bax.
Figure 5.
GT-induced cytochrome c release from mitochondria is dependent on Bak and the ROS generation. (A) wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− MEFs were incubated with (red) or without (black) 1 μM GT for 4 h and analyzed by FACS for mitochondrial release of cytochrome c using a α–cytochrome c mAb followed by a FITC-labeled secondary antibody. Numbers depicted are the percentages of cells negative for cytochrome c (indicated by horizontal bars). (B) Mitochondria isolated from wt, Bax−/−, Bak−/−, and Bak−/− × Bax−/− FDMs (left) or MEFs (right) were treated with 10, 20, or 50 μM of GT or 40 nM of tBid for 4 h in vitro. After centrifugation, cytochrome c was measured in mitochondria and the supernatant by α–cytochrome c Western blotting (i). In parallel, the presence of Bak and Bax was analyzed in MEF wt, Bax−/−, and Bak−/− by simultaneous α-Bax and α-Bak immunoblotting of the mitochondrial preparations. As control, an antibody against the α subunit of the H+-ATPase was used (ii). (C) wt MEF cells were incubated with (red) or without (black) 1 μM GT for 4 h in the presence or absence of 100 μM ZVAD-fmk (green) or 15 mM NAC (blue) and analyzed by FACS for mitochondrial release of cytochrome c as in A. Data shown are representatives of at least three independent experiments with similar outcome.
Figure 6.
GT-induced AIF translocation to the nucleus is dependent on Bak and the generation of ROS. (A) wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− MEF cells were incubated with or without 1 μM GT for 4 h and analyzed by confocal microscopy for nuclear translocation of AIF using a specific α-AIF rabbit antibody. Numbers depicted are the percentages of cells with nuclear AIF. (B) Alternatively, wt MEFs were incubated with or without 1 μM of GT for 4 h in the presence or absence of 100 μM ZVAD-fmk or 15 mM NAC and analyzed for nuclear translocation of AIF as described above. Data are representative of two independent experiments. Bars, 10 μm.
Consistent with a direct action of GT on mitochondrial membrane permeability, GT-induced cytochrome c and AIF release was not blocked by ZVAD in wt MEFs (Fig. 5 C and Fig. 6 B). Most important, however, both cytochrome c and AIF release from mitochondria was greatly diminished by NAC (Fig. 5 C and Fig. 6 B). This suggests that GT-induced ROS production is crucial for effective release of apoptogenic factors from mitochondria but does not exclude the possibility that NAC has some other mitochondrial membrane stabilizing activity.
Bak-deficient mice are resistant to IA
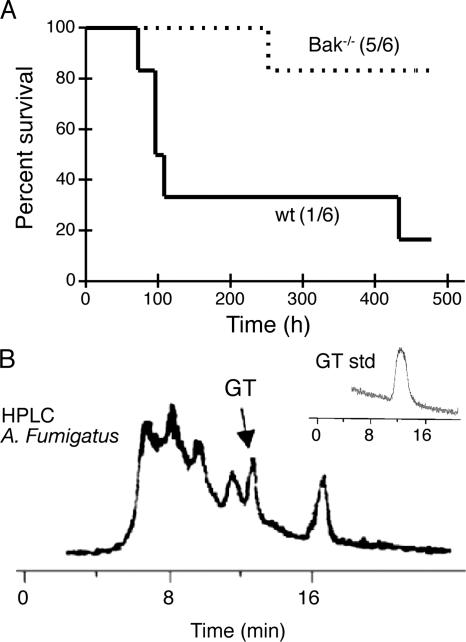
To determine whether the selective activation of Bak during GT-mediated cell death as seen in our in vitro analysis is of biological relevance and pathophysiological significance, we monitored the mortality of immunosuppressed (hydrocortisone-treated) wt (C57BL/6) and Bak ko (Bak−/−) mice subsequently infected with a GT-producing A. fumigatus strain. The presence of GT synthesis by this fungal strain was verified by HPLC analysis of culture SN (Fig. 7 B). Fig. 7 A shows that 5 out of 6 wt mice died within 2 wk after intranasal infection, and only 1 out of 6 Bak−/− mice succumbed over the same time period (P < 0.015). These in vivo data correlate with our in vitro findings and show for the first time that Bak is a host susceptibility factor for A. fumigatus virulence in mice, probably because of its direct activation by GT.
Figure 7.
Deletion of Bak results in resistance to mice of A. fumigatus. (A) Bak−/− mice are resistant to A. fumigatus infection. Female wt C57BL6 or Bak−/− mice (6 mice per group) were immunosuppressed by subcutaneous injection of 2 mg of hydrocortisone in PBS/0.1% Tween-20 on days −4, −2, 0, 2, and 4 of infection. On day 0, recipients were inoculated intranasally with 5 × 106 A. fumigatus B5233 in 20 μl of PBS or with 20 μl PBS alone (control), and morbidity and mortality was monitored during the time. Control mice survive without any symptoms of infection. Survival was significantly different (P < 0.015), as analyzed by the Kaplan-Meier survival test. (B) A. fumigatus B5233 is able to produce GT. 107 A. fumigatus spores were inoculated in 100 ml of RPMI and grown for 48 h at 37°C and 0.5% CO2, and the presence of GT in the SN (black) was assessed by HPLC, using a GT standard (inset) as described in Materials and methods.
Discussion
Although GT has long been proposed to constitute a virulence factor in IA (Eichner and Mullbacher, 1984; Mullbacher and Eichner, 1984), most probably by suppressing immune responses via induction of mammalian cell apoptosis (Waring et al., 1988b; Sutton et al., 1994), the molecular mechanisms underlying the putative in vitro and in vivo processes have not been elucidated. Here, we present evidence that Bak, but not Bax, is a key host factor in GT-mediated cell death in vitro. We used MEFs and FDMs deficient in the Bcl-2 family members Bak and/or Bax or their activator Bid (Wei et al., 2000) and isolated mitochondria from these cells to show that GT-mediated activation of Bak occurs independently of Bid or other cytosolic factors. Once activated, Bak triggers the generation of ROS, which is crucial for effective mitochondrial membrane pore formation, including the release of cytochrome c and AIF, and ultimate cell death. The additional finding that the virulence of GT-producing A. fumigatus was significantly decreased in Bak−/− over wt mice strongly implicates that GT is an important modulator in mammalian host defense and that Bak is a prominent host susceptibility factor. The interrelation of GT and Bak in A. fumigatus pathology is also supported by the recent finding that an A. fumigatus mutant lacking GT expresses a drastically reduced virulence (unpublished data).
At present, it is unclear how GT activates Bak. One mechanism by which GT may activate Bak is by breaking up inhibitory complexes between Bak and Bcl-2–like prosurvival factors (Sattler et al., 1997; Cuconati et al., 2003; Willis et al., 2005; Ekert et al., 2006) or VDAC2 (Cheng et al., 2003) on the mitochondrial membrane. This could be by forming transient disulphide bonds between the reactive disulphide bond in GT and individual cysteine residues in Bak or its binding partners, leading to the release of active Bak. Three-dimensional structures of Bak–Bcl-2 or Bak–VDAC2 complexes have not yet been determined, so we do not know if any cysteine residues are involved in these interactions. Moreover, we do not have any experimental evidence that GT directly binds Bak, Bcl-2–like proteins, or VDAC2. However, our data obtained with isolated mitochondria favor an interpretation that GT-facilitated activation of Bak occurs by direct interaction with antiapoptotic Bcl-2 family members or other mitochondrial membrane–associated constituents. The former possibility is supported by the observation that protection against GT-mediated monocyte apoptosis by agonists of nerve growth factor receptors is associated with the up-regulation of Bcl-2 and Bcl-xL (la Sala et al., 2000).
The finding that GT specifically acts through Bak and not Bax is intriguing. Although both proteins are supposed to exert the same pore-forming activity on mitochondria (Kuwana et al., 2005), they are activated differently. There is increasing evidence for selective Bax- or Bak-specific apoptosis, depending on the cell type and the apoptotic stimuli (Lindenboim et al., 2005; Wendt et al., 2005). Thus, the activation mechanism of Bax and Bak may be distinct, although both ultimately oligomerize and form pores in the outer mitochondrial membrane. In this respect, GT may be unable to interact with Bax and/or to release any of its inhibitory components. Moreover, an interaction of GT with Bcl-2 or Bcl-xL would not affect Bax because it is not sequestered by these proteins in healthy cells. This would explain why GT induces conformational activation of Bak but not of Bax.
The findings that the mitochondrial protein VDAC2 associates with and inhibits Bak in healthy mitochondria (Cheng et al., 2003) and that in monocytes, Bak but not Bax is part of the VDAC channel (unpublished data) suggest VDAC2's involvement in GT-mediated cell death. VDAC2 is one of three mammalian isoforms of VDAC proteins (VDAC1, -2, and -3), which constitute the major pathway for metabolic exchange across the outer mitochondrial membrane (Sampson et al., 1997; Wu et al., 1999; Xu et al., 1999). Together with cyclophilin D and adenine nucleotide transporter (ANT), VDAC forms the mitochondrial permeability transition pore (MPTP), involved in cell apoptosis and/or necrosis (Crompton, 1999; Zheng et al., 2004). How the function of MPTP is regulated by members of the BH3 family is still highly controversial (Marzo et al., 1998; Shimizu et al., 1999; Vander Heiden et al., 1999). One could postulate that GT somehow modulates the VDAC complex, leading to the liberation of Bak, a subsequent increase of mitochondrial membrane permeability and hence a Bak-dependent cytochrome c release and cell death. The contribution of the MPTP in the latter process is further supported by the findings that GT-induced apoptosis of activated hepatic stellate cells is associated with a specific thiol redox-dependent interaction with MPTP component ANT (Orr et al., 2004) and that cyclosporin A, an inhibitor of cyclophilin D and mitochondrial pore opening (Crompton et al., 1988), affected mitochondrial depolarization and ROS production (Kweon et al., 2003; unpublished data). Most notable, the data suggested that oxidative cross-linking of two matrix-facing cysteine residues on the ANT (Cys56 and Cys159) plays a key role in regulating the MPTP (Halestrap et al., 2002). However, more detailed studies, including MPTP inhibitors such as bongkrekic acid or cyclosporins A, are required to dissect the role of the VDAC–ANT complex in GT-mediated and Bak-dependent cell death.
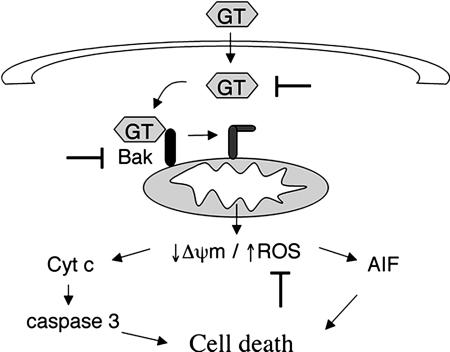
Our data further show that GT-induced production of ROS is mandatory for cell death. The sequence of events leading to ROS production by GT was revealed by analyzing the various proapoptotic processes in the presence of inhibitors for ROS and for caspases, including NAC, MnTBAP, and ZVAD-fmk, respectively (Fig. 8). Accordingly, activation of Bak precedes the generation of ROS, which then facilitate the release of cytochrome c and AIF from mitochondria, leading to caspase activation as well as mitochondria- and caspase-independent events to mediate cell death. As to the source of ROS, it is possible that they are generated from a perturbance of mitochondrial respiration that is due to Bak-mediated pore formation and/or activation of MPTP. Why ROS are, at least partially, required for cytochrome c release is unclear, although it has been shown that ROS generation is crucial for cytochrome c release under different apoptotic stimuli (Petrosillo et al., 2001; Orrenius and Zhivotovsky, 2005; Santamaria et al., 2006).
Figure 8.
Proposed mechanism for GT-induced cell death and aspergillosis treatment. After production by A. fumigatus, GT would enter cells by a redox-dependent mechanism and directly induce a conformational change in Bak, leading to mitochondrial depolarization and ROS production. ROS production then triggers the mitochondrial release of apoptogenic proteins such as cytochrome c (cyt c) and AIF. In this way, both caspase-dependent and -independent processes would be launched to induce cell death. By blocking either GT or Bak conformational change or even ROS production, GT-induced cell death could be prevented and the damage exerted by A. fumigatus attenuated.
The putative relevance of GT-mediated apoptosis for the parasitized vertebrate host was analyzed by comparing the course of A. fumigatus infection in wt and Bak−/− mice. The significantly decreased virulence of the pathogen observed in Bak−/− as compared with wt mice, as revealed by the differential kinetics of mortality, supports the following assumptions: GT is released during A. fumigatus infection, as suggested before (Eichner and Mullbacher, 1984; Lewis et al., 2005), and induces apoptosis in multiple target cells, most probably via Bak activation. This process subsequently leads to an accelerated colonization of target organs by breaching physical barriers, such as lung and renal epithelial cells, and establishes an immunosuppressed state of the host. Although the present data do not formally proof a cause–effect relationship between GT, Bak activation, and A. fumigatus pathogenicity (virulence), the previous (Eichner and Mullbacher, 1984; Mullbacher and Eichner, 1984) and present assumption that GT is a virulence factor of A. fumigatus is supported by a recent report (Bok et al., 2005) and our own unpublished data. These results have shown that low levels of pulmonary GT observed with an A. fumigatus mutant defective in LaeA, a global regulator of secondary metabolism, is associated with impaired virulence of the pathogen. Furthermore, by using a recently generated glip gene knockout mutant of A. fumigatus lacking GT, we found that this mutant is much less virulent in mice than the wt strain and that cell culture supernatants were unable to induce cell death (unpublished data).
Based on the sequence of intracellular events occurring during GT-induced apoptosis (Fig. 8), we conclude that GT is a critical virulence factor in A. fumigatus. This is supported by the fact that GT is one of the most abundant secondary metabolites produced by the fungus (Taylor, 1971) and that Bak−/− mice are more resistant to infection by A. fumigatus. The distinct potential of GT to activate Bak, but not Bax, may be of relevance for the development of anti-IA drugs that selectively block cell death pathways via Bak and, at the same time, spare the residual proapoptotic proteins relevant for the control of the pathogen by the host's immune system.
Materials and methods
Cell culture and reagents
SV40 transformed MEFs (Wei et al., 2001) and MBL-2–Fas cells were cultured in MEM supplemented with 10% FCS and 2-mercaptoethanol (10−5 M) at 37°C and 7% CO2. The IL-3–dependent (FDM) cell lines were generated by coculturing embryonic day 14.4 fetal liver single-cell suspensions with fibroblasts expressing a HoxB8 retrovirus in the presence of high IL-3 concentrations, as previously described (Ekert et al., 2004). Bak−/− mice were obtained from C. Thompson (Harvard Medical School, Boston, MA), backcrossed for nine generations to ensure a “pure” C57BL/6 genetic background, and intercrossed with Bax−/− C57BL/6 mice to obtain Bax−/− × Bak−/− mice (provided by D. Huang, The Walter and Eliza Hall Institute, Melbourne, Australia) as described previously (Willis et al., 2005). The cell lines were cultured in MEM with Earle's salts and l-glutamine. For Western blot analysis of isolated mitochondria, antibodies to H+-ATPase (Invitrogen) were used as controls.
GT was purified from Penicillium terlikowskii as described previously (Waring et al., 1988b). The purity of this preparation was analyzed by TLC and HPLC showing the same quality as commercial GT. For apoptosis induction, 2 × 105 cells/ml MEFs were incubated with the indicated concentration of GT or staurosporine (Sigma-Aldrich) for 4 h, and apoptosis assays were performed as described in the following paragraphs. In some cases, the general caspase inhibitor Ac-ZVAD-fmk (Bachem) or the ROS scavengers NAC (Sigma-Aldrich) or MnTBAP (Calbiochem) were added as described previously (Pardo et al., 2004). To test the inhibitory potency of Ac-ZVAD-fmk, MBL-2–Fas cells were incubated with 1 μg/ml α-Fas mAb Jo-2 for 24 h in the presence or absence of 100 μM of the caspase inhibitor, and cell death was analyzed by trypan blue exclusion. Nuclei were stained with 10 μg/ml Hoechst 33342 (Invitrogen).
Nuclear, cell membrane, and mitochondrial membrane perturbation
PS exposure and PI uptake was analyzed by FACS or fluorescence microscopy as described previously (Pardo et al., 2004) using the annexin V–FITC kit from BD Biosciences. Δψm was measured with the fluorescent probe 3,3′-dihexyloxacarbocyanine iodide (DiOC6[3]; Invitrogen) and ROS generation with 2-hydroxiethidine (2-HE; Invitrogen) as described previously (Pardo et al., 2004). Nuclear morphology was analyzed by fluorescence microscopy with Hoechst 33342. For that, cells were fixed with 1% PFA and mounted on a drop of Fluoromount-G (Southern Biotechnology Associates, Inc.) containing 10 μg/ml Hoechst 33342, and images were taken at room temperature using a microscope (Axioskop 10; Carl Zeiss MicroImaging, Inc.), an analysis camera (Axiocam; Carl Zeiss MicroImaging, Inc.), and Vision 3.1.0.0 software (Carl Zeiss MicroImaging, Inc.). The objective used was a PlanNeofluor (Carl Zeiss MicroImaging, Inc.), with a magnification of 40 and a NA of 0.75. Photoshop CS2 (Adobe) was used for minor adjustments to contrast and image overlay. In some cases, membrane perturbation was also analyzed by fluorescence microscopy by staining the cells with the annexin V–FITC kit from BD Biosciences. Images were taken in the same conditions as described above, but cells were mounted on annexin binding buffer (BD Biosciences).
Caspase-3 activation
Cells were fixed with 2.5% PFA, incubated with a mAb FITC-labeled against the active form of caspase-3 (clone C92605; BD Biosciences), and analyzed by FACS as described previously (Pardo et al., 2004).
Cytochrome c release and nuclear translocation of AIF on cells
Cytochrome c release was quantified by FACS analysis as recently described (Waterhouse and Trapani, 2003). In brief, 106 MEFs were mildly permeabilized with 25 μg/ml digitonin plus 100 mM KCl on ice for 5 min. This led to the cellular loss of cytosolic cytochrome c. Cells were washed once with cold PBS, fixed in 4% PFA, permeabilized with 0.05% saponin and 3% BSA, and incubated with the α–cytochrome c mAb 6H2.B4 (BD Biosciences) or mouse IgG isotype control (Jackson ImmunoResearch Laboratories) followed by α-mouse-FITC secondary antibody (Jackson ImmunoResearch Laboratories). The cells were resuspended in 100 μl PFA in PBS and analyzed by FACS with a FACScan (BD Biosciences) and CellQuest software (BD Biosciences). For the analysis of the nuclear translocation of AIF, cells were fixed, mounted on poly-l-lysine cover slides, stained with a rabbit polyclonal α-AIF antibody (Sigma-Aldrich) followed by the secondary goat α-rabbit antibody labeled with Alexa 488 as described previously (Pardo et al., 2001), and mounted on a drop of Fluoromount-G. Afterward, the cells were analyzed by confocal microscopy. Fluorescence images were taken at room temperature on a confocal microscope (TCS SP2; Leica) using a 40× objective (HCX PL APO CS; Leica), NA 1.25, immersion oil, and confocal software (version 2.61; Leica). Photoshop CS2 was used for minor adjustments to contrast.
Cytochrome c release from isolated mitochondria
8 × 107 MEFs or FDMs were centrifuged and washed once in PBS. The cell pellets were resuspended in 500 μl MSH buffer (210 mM mannitol, 70 mM sucrose, 20 mM Hepes, 1 mM EDTA, pH 7.5, 100 μM PMSF, 400 ng/ml pepstatin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 5 μg/ml cytochalasin B). The resuspended cell pellet was incubated on ice for 15 min before the cells were broken by passaging 25 times through a 23-gauge needle. The lysate was centrifuged at 500 g for 5 min to remove cell debris and nuclei. A crude mitochondrial pellet was then obtained by centrifugation at 10,000 g for 15 min and resuspended in MSH buffer. The isolated mitochondria were incubated with different concentrations of GT (10, 20, and 50 μM) or 40 nM of recombinant tBid (provided by J.C. Martinou, University of Geneva, Geneva, Switzerland) as a positive control at 37°C for 4 h. After incubation, the mitochondria were pelleted, and both pellet and supernatant were tested for cytochrome c release by SDS-PAGE.
Conformational change of Bax and Bak
MEFs were fixed in 4% PFA, permeabilized with 0.1% saponin in PBS/5% FCS, and incubated with 2 μg/ml rabbit polyclonal α-Bak (NT; Upstate Biotechnology), 5 μg/ml rabbit polyclonal α-Bax (NT; Upstate Biotechnology), or 5 μg/ml rabbit purified IgG (control). After two washes with 0.1% saponin in PBS, the cells were incubated with α-rabbit-FITC antibody in 0.1% saponin/PBS/5% FCS, washed twice in 0.1% saponin/PBS, resuspended in 1% PFA/PBS, and analyzed by FACS with a FACScan and CellQuest software. The amount of Bax and Bak on isolated mitochondria from wt, Bak−/−, Bax−/−, and Bak−/− × Bax−/− FDMs was determined by washing the centrifuged mitochondria twice in large amounts MSH buffer (to eliminate cytosolic contamination) followed by lysing the mitochondria in SDS sample buffer and analysis by α-Bax (Bax-NT) and α-Bak (Bak-NT) Western blotting on the same gel. As mitochondrial marker and loading control, an antibody against the F0F1 ATPase was used.
In vivo invasive aspergillosis model and GT analysis on culture supernatants
Female mice (C57BL/6, Bak−/− [Jackson ImmunoResearch Laboratories; C57BL/6.129, six times backcrossed in C57BL/6], or 129/Sv) were immunosuppressed by subcutaneous injection of 3 mg (112 mg/kg) of hydrocortisone (Sigma-Aldrich) diluted in 200 μl of PBS/0.1% Tween 20 on days −4, −2, 0, 2, and 4, as described previously (Tang et al., 1993). On day 0, mice (6 per group) were infected intranasally with 5 × 106 A. fumigatus B5233 conidia in 20 μl of PBS or with PBS alone. Disease development was analyzed by morbidity/mortality of the mice after infection. There was no difference in the sensitivity of C57BL/6 or 129/Sv mice to A. fumigatus infection, and all infected recipients of both mouse strains died during the first week after infection. GT presence on A. fumigatus B5233 culture supernatants was analyzed after 48 h by HPLC as described previously (Belkacemi et al., 1999).
Acknowledgments
We thank D. Huang for the Bax−/− and the Bax−/− × Bak−/− mice, C. Thompson for the Bak−/− mice, the S.J. Korsmeyer Laboratory for providing the Bid−/−, Bax−/−, Bak−/−, and Bax−/− × Bak−/− MEFs, J.C. Martinou for recombinant tBid, P. Martin for help in in vivo infections, R. Lamers for critical comments, and P. Graeber for allowing us to use his HPLC equipment.
J. Pardo was supported by the Alexander von Humboldt Foundation, C. Borner and E.M. Galvez by the Deutsche Forschungsgesellschaft (BO-1933 to C. Borner and 1302/1-1 to E.M. Galvez), J. Kwon-Chung by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, and P.G. Ekert by an National Health and Medical Research Council career development grant.
The authors declare no competing financial interests.
Abbreviations used in this paper: 2-HE, 2-hydroxiethidine; AIF, apoptosis-inducing factor; ANT, adenine nucleotide transporter; FDM, factor-dependent myeloid; GT, gliotoxin; IA, invasive aspergilloses; MEF, mouse embryonic fibroblast; MnTBAP, Mn(III) tetrakis(4-benzoic acid) porphyrin chloride; MPTP, mitochondrial permeability transition pore; NAC, N-acetylcysteine; PI, propidium iodide; PS, phosphatidylserine; ROS, reactive oxygen species; wt, wild-type.
References
- Bauters, T.G., F.M. Buyle, R. Peleman, and H. Robays. 2005. Antifungal drugs and rational use of antifungals in treating invasive aspergillosis: the role of the hospital pharmacist. Pharm. World Sci. 27:31–34. [DOI] [PubMed] [Google Scholar]
- Belkacemi, L., R.C. Barton, V. Hopwood, and E.G. Evans. 1999. Determination of optimum growth conditions for gliotoxin production by Aspergillus fumigatus and development of a novel method for gliotoxin detection. Med. Mycol. 37:227–233. [PubMed] [Google Scholar]
- Bernardo, P.H., N. Brasch, C.L. Chai, and P. Waring. 2003. A novel redox mechanism for the glutathione-dependent reversible uptake of a fungal toxin in cells. J. Biol. Chem. 278:46549–46555. [DOI] [PubMed] [Google Scholar]
- Bok, J.W., S.A. Balajee, K.A. Marr, D. Andes, K.F. Nielsen, J.C. Frisvad, and N.P. Keller. 2005. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell. 4:1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite, A.W., R.D. Eichner, P. Waring, and A. Mullbacher. 1987. The immunomodulating agent gliotoxin causes genomic DNA fragmentation. Mol. Immunol. 24:47–55. [DOI] [PubMed] [Google Scholar]
- Chandra, J., A. Samali, and S. Orrenius. 2000. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 29:323–333. [DOI] [PubMed] [Google Scholar]
- Chen, L., S.N. Willis, A. Wei, B.J. Smith, J.I. Fletcher, M.G. Hinds, P.M. Colman, C.L. Day, J.M. Adams, and D.C. Huang. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 17:393–403. [DOI] [PubMed] [Google Scholar]
- Cheng, E.H., T.V. Sheiko, J.K. Fisher, W.J. Craigen, and S.J. Korsmeyer. 2003. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 301:513–517. [DOI] [PubMed] [Google Scholar]
- Crompton, M. 1999. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Crompton, M., H. Ellinger, and A. Costi. 1988. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 255:357–360. [PMC free article] [PubMed] [Google Scholar]
- Cuconati, A., C. Mukherjee, D. Perez, and E. White. 2003. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17:2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial, N.N., and S.J. Korsmeyer. 2004. Cell death: critical control points. Cell. 116:205–219. [DOI] [PubMed] [Google Scholar]
- Day, B.J., I. Fridovich, and J.D. Crapo. 1997. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 347:256–262. [DOI] [PubMed] [Google Scholar]
- Dockrell, D.H. 2001. Apoptotic cell death in the pathogenesis of infectious diseases. J. Infect. 42:227–234. [DOI] [PubMed] [Google Scholar]
- Eichner, R.D., and A. Mullbacher. 1984. Hypothesis: fungal toxins are involved in aspergillosis and AIDS. Aust. J. Exp. Biol. Med. Sci. 62:479–484. [DOI] [PubMed] [Google Scholar]
- Eichner, R.D., M. Al Salami, P.R. Wood, and A. Mullbacher. 1986. The effect of gliotoxin upon macrophage function. Int. J. Immunopharmacol. 8:789–797. [DOI] [PubMed] [Google Scholar]
- Eichner, R.D., P. Waring, A.M. Geue, A.W. Braithwaite, and A. Mullbacher. 1988. Gliotoxin causes oxidative damage to plasmid and cellular DNA. J. Biol. Chem. 263:3772–3777. [PubMed] [Google Scholar]
- Ekert, P.G., S.H. Read, J. Silke, V.S. Marsden, H. Kaufmann, C.J. Hawkins, R. Gerl, S. Kumar, and D.L. Vaux. 2004. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J. Cell Biol. 165:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert, P.G., A.M. Jabbour, A. Manoharan, J.E. Heraud, J. Yu, M. Pakusch, E.M. Michalak, P.N. Kelly, B. Callus, T. Kieffer, et al. 2006. Cell death provoked by loss of interleukin-3 signalling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 10.1182/blood-2006-03-014209. [DOI] [PubMed]
- Faulkner, K.M., R.D. Stevens, and I. Fridovich. 1994. Characterization of Mn(III) complexes of linear and cyclic desferrioxamines as mimics of superoxide dismutase activity. Arch. Biochem. Biophys. 310:341–346. [DOI] [PubMed] [Google Scholar]
- Gardiner, D.M., P. Waring, and B.J. Howlett. 2005. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology. 151:1021–1032. [DOI] [PubMed] [Google Scholar]
- Green, D.R., and G. Kroemer. 2004. The pathophysiology of mitochondrial cell death. Science. 305:626–629. [DOI] [PubMed] [Google Scholar]
- Griffiths, G.J., L. Dubrez, C.P. Morgan, N.A. Jones, J. Whitehouse, B.M. Corfe, C. Dive, and J.A. Hickman. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap, A.P., G.P. McStay, and S.J. Clarke. 2002. The permeability transition pore complex: another view. Biochimie. 84:153–166. [DOI] [PubMed] [Google Scholar]
- Hsu, Y.T., K.G. Wolter, and R.J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA. 94:3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D.C., and A. Strasser. 2000. BH3-only proteins—essential initiators of apoptotic cell death. Cell. 103:839–842. [DOI] [PubMed] [Google Scholar]
- Hurne, A.M., C.L. Chai, K. Moerman, and P. Waring. 2002. Influx of calcium through a redox-sensitive plasma membrane channel in thymocytes causes early necrotic cell death induced by the epipolythiodioxopiperazine toxins. J. Biol. Chem. 277:31631–31638. [DOI] [PubMed] [Google Scholar]
- Kroemer, G., and J.C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513–519. [DOI] [PubMed] [Google Scholar]
- Kuwana, T., L. Bouchier-Hayes, J.E. Chipuk, C. Bonzon, B.A. Sullivan, D.R. Green, and D.D. Newmeyer. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 17:525–535. [DOI] [PubMed] [Google Scholar]
- Kweon, Y.O., Y.H. Paik, B. Schnabl, T. Qian, J.J. Lemasters, and D.A. Brenner. 2003. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J. Hepatol. 39:38–46. [DOI] [PubMed] [Google Scholar]
- la Sala, A., S. Corinti, M. Federici, H.U. Saragovi, and G. Girolomoni. 2000. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J. Leukoc. Biol. 68:104–110. [PubMed] [Google Scholar]
- Latge, J.P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge, J.P. 2001. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 9:382–389. [DOI] [PubMed] [Google Scholar]
- Letai, A., M.C. Bassik, L.D. Walensky, M.D. Sorcinelli, S. Weiler, and S.J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2:183–192. [DOI] [PubMed] [Google Scholar]
- Lewis, R.E., N.P. Wiederhold, J. Chi, X.Y. Han, K.V. Komanduri, D.P. Kontoyiannis, and R.A. Prince. 2005. Detection of gliotoxin in experimental and human aspergillosis. Infect. Immun. 73:635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. [DOI] [PubMed] [Google Scholar]
- Lindenboim, L., S. Kringel, T. Braun, C. Borner, and R. Stein. 2005. Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 12:713–723. [DOI] [PubMed] [Google Scholar]
- Lindsten, T., A.J. Ross, A. King, W.X. Zong, J.C. Rathmell, H.A. Shiels, E. Ulrich, K.G. Waymire, P. Mahar, K. Frauwirth, et al. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 6:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., C.N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 86:147–157. [DOI] [PubMed] [Google Scholar]
- Longthorne, V.L., and G.T. Williams. 1997. Caspase activity is required for commitment to Fas-mediated apoptosis. EMBO J. 16:3805–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo, I., C. Brenner, and G. Kroemer. 1998. The central role of the mitochondrial megachannel in apoptosis: evidence obtained with intact cells, isolated mitochondria, and purified protein complexes. Biomed. Pharmacother. 52:248–251. [DOI] [PubMed] [Google Scholar]
- Mullbacher, A., and R.D. Eichner. 1984. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc. Natl. Acad. Sci. USA. 81:3835–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher, A., P. Waring, and R.D. Eichner. 1985. Identification of an agent in cultures of Aspergillus fumigatus displaying anti-phagocytic and immunomodulating activity in vitro. J. Gen. Microbiol. 131:1251–1258. [DOI] [PubMed] [Google Scholar]
- Mullbacher, A., P. Waring, U. Tiwari-Palni, and R.D. Eichner. 1986. Structural relationship of epipolythiodioxopiperazines and their immunomodulating activity. Mol. Immunol. 23:231–235. [DOI] [PubMed] [Google Scholar]
- Mullbacher, A., D. Hume, A.W. Braithwaite, P. Waring, and R.D. Eichner. 1987. Selective resistance of bone marrow-derived hemopoietic progenitor cells to gliotoxin. Proc. Natl. Acad. Sci. USA. 84:3822–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama, T., R. Amitani, Y. Ikegami, R. Nawada, W.J. Lee, and F. Kuze. 1996. Suppressive effects of Aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur. Respir. J. 9:293–300. [DOI] [PubMed] [Google Scholar]
- Nechushtan, A., C.L. Smith, Y.T. Hsu, and R.J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, J.G., V. Leel, G.A. Cameron, C.J. Marek, E.L. Haughton, L.J. Elrick, J.E. Trim, G.M. Hawksworth, A.P. Halestrap, and M.C. Wright. 2004. Mechanism of action of the antifibrogenic compound gliotoxin in rat liver cells. Hepatology. 40:232–242. [DOI] [PubMed] [Google Scholar]
- Orrenius, S., and B. Zhivotovsky. 2005. Cardiolipin oxidation sets cytochrome c free. Nat. Chem. Biol. 1:188–189. [DOI] [PubMed] [Google Scholar]
- Pahl, H.L., B. Krauss, K. Schulze-Osthoff, T. Decker, E.B. Traenckner, M. Vogt, C. Myers, T. Parks, P. Warring, A. Muhlbacher, et al. 1996. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-κB. J. Exp. Med. 183:1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, J., P. Perez-Galan, S. Gamen, I. Marzo, I. Monleon, A.A. Kaspar, S.A. Susin, G. Kroemer, A.M. Krensky, J. Naval, and A. Anel. 2001. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J. Immunol. 167:1222–1229. [DOI] [PubMed] [Google Scholar]
- Pardo, J., A. Bosque, R. Brehm, R. Wallich, J. Naval, A. Mullbacher, A. Anel, and M.M. Simon. 2004. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J. Cell Biol. 167:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo, G., F.M. Ruggiero, M. Pistolese, and G. Paradies. 2001. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 509:435–438. [DOI] [PubMed] [Google Scholar]
- Sampson, M.J., R.S. Lovell, and W.J. Craigen. 1997. The murine voltage-dependent anion channel gene family. Conserved structure and function. J. Biol. Chem. 272:18966–18973. [DOI] [PubMed] [Google Scholar]
- Santamaria, G., M. Martinez-Diez, I. Fabregat, and J.M. Cuezva. 2006. Efficient execution of cell death in non-glycolytic cells requires the generation of ROS controlled by the activity of mitochondrial H+-ATP synthase. Carcinogenesis. 27:925–935. [DOI] [PubMed] [Google Scholar]
- Sattler, M., H. Liang, D. Nettesheim, R.P. Meadows, J.E. Harlan, M. Eberstadt, H.S. Yoon, S.B. Shuker, B.S. Chang, A.J. Minn, et al. 1997. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 275:983–986. [DOI] [PubMed] [Google Scholar]
- Schweizer, M., and C. Richter. 1994. Gliotoxin stimulates Ca2+ release from intact rat liver mitochondria. Biochemistry. 33:13401–13405. [DOI] [PubMed] [Google Scholar]
- Shah, D.T., S. Jackman, J. Engle, and B. Larsen. 1998. Effect of gliotoxin on human polymorphonuclear neutrophils. Infect. Dis. Obstet. Gynecol. 6:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, S., M. Narita, and Y. Tsujimoto. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 399:483–487. [DOI] [PubMed] [Google Scholar]
- Suen, Y.K., K.P. Fung, C.Y. Lee, and S.K. Kong. 2001. Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic. Res. 35:1–10. [DOI] [PubMed] [Google Scholar]
- Susin, S.A., H.K. Lorenzo, N. Zamzami, I. Marzo, B.E. Snow, G.M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, et al. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 397:441–446. [DOI] [PubMed] [Google Scholar]
- Sutton, P., N.R. Newcombe, P. Waring, and A. Mullbacher. 1994. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 62:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, C.M., J. Cohen, T. Krausz, S. Van Noorden, and D.W. Holden. 1993. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect. Immun. 61:1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. 1971. The toxicology of sporidesmins and other epipolythiadioxopiperazines. In Microbial Toxins, vol. 7. S. Kadis, A. Ciegler, and S.J. Ajl, editors. Academic Press, New York. 337–376.
- Tsunawaki, S., L.S. Yoshida, S. Nishida, T. Kobayashi, and T. Shimoyama. 2004. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 72:3373–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden, M.G., N.S. Chandel, P.T. Schumacker, and C.B. Thompson. 1999. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell. 3:159–167. [DOI] [PubMed] [Google Scholar]
- Waring, P., R.D. Eichner, and A. Mullbacher. 1988. a. The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med. Res. Rev. 8:499–524. [DOI] [PubMed] [Google Scholar]
- Waring, P., R.D. Eichner, A. Mullbacher, and A. Sjaarda. 1988. b. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J. Biol. Chem. 263:18493–18499. [PubMed] [Google Scholar]
- Waring, P., N. Newcombe, M. Edel, Q.H. Lin, H. Jiang, A. Sjaarda, T. Piva, and A. Mullbacher. 1994. Cellular uptake and release of the immunomodulating fungal toxin gliotoxin. Toxicon. 32:491–504. [DOI] [PubMed] [Google Scholar]
- Waterhouse, N.J., and J.A. Trapani. 2003. A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Differ. 10:853–855. [DOI] [PubMed] [Google Scholar]
- Wei, M.C., T. Lindsten, V.K. Mootha, S. Weiler, A. Gross, M. Ashiya, C.B. Thompson, and S.J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei, M.C., W.X. Zong, E.H. Cheng, T. Lindsten, V. Panoutsakopoulou, A.J. Ross, K.A. Roth, G.R. MacGregor, C.B. Thompson, and S.J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt, J., C. von Haefen, P. Hemmati, C. Belka, B. Dorken, and P.T. Daniel. 2005. TRAIL sensitizes for ionizing irradiation-induced apoptosis through an entirely Bax-dependent mitochondrial cell death pathway. Oncogene. 24:4052–4064. [DOI] [PubMed] [Google Scholar]
- Wichmann, G., O. Herbarth, and I. Lehmann. 2002. The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol. 17:211–218. [DOI] [PubMed] [Google Scholar]
- Willis, S.N., L. Chen, G. Dewson, A. Wei, E. Naik, J.I. Fletcher, J.M. Adams, and D.C. Huang. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S., M.J. Sampson, W.K. Decker, and W.J. Craigen. 1999. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim. Biophys. Acta. 1452:68–78. [DOI] [PubMed] [Google Scholar]
- Xu, X., W. Decker, M.J. Sampson, W.J. Craigen, and M. Colombini. 1999. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J. Membr. Biol. 170:89–102. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Y. Shi, C. Tian, C. Jiang, H. Jin, J. Chen, A. Almasan, H. Tang, and Q. Chen. 2004. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 23:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., A. Zhao, G. Goping, and P. Hirszel. 2000. Gliotoxin-induced cytotoxicity proceeds via apoptosis and is mediated by caspases and reactive oxygen species in LLC-PK1 cells. Toxicol. Sci. 54:194–202. [DOI] [PubMed] [Google Scholar]