Abstract
Reduced homeostatic capacity for intracellular Ca2+ ([Ca2+]i) movement may underlie the progression of sarcopenia and contractile dysfunction during muscle aging. We report two alterations to Ca2+ homeostasis in skeletal muscle that are associated with aging. Ca2+ sparks, which are the elemental units of Ca2+ release from sarcoplasmic reticulum, are silent under resting conditions in young muscle, yet activate in a dynamic manner upon deformation of membrane structures. The dynamic nature of Ca2+ sparks appears to be lost in aged skeletal muscle. Using repetitive voltage stimulation on isolated muscle preparations, we identify a segregated [Ca2+]i reserve that uncouples from the normal excitation–contraction process in aged skeletal muscle. Similar phenotypes are observed in adolescent muscle null for a synaptophysin-family protein named mitsugumin-29 (MG29) that is involved in maintenance of muscle membrane ultrastructure and Ca2+ signaling. This finding, coupled with decreased expression of MG29 in aged skeletal muscle, suggests that MG29 expression is important in maintaining skeletal muscle Ca2+ homeostasis during aging.
Introduction
Effective muscle contractile performance is contingent upon the maintenance of Ca2+ homeostasis and signaling, which requires that intracellular Ca2+ ([Ca2+]i) be readily available for release. In skeletal muscle, excitation–contraction (E–C) coupling is primarily mediated by conformational coupling between voltage sensors of the sarcolemmal membrane and ryanodine receptor (RyR) Ca2+ release channel of the sarcoplasmic reticulum (SR; Ma et al., 1988; Rios et al., 1992; Franzini-Armstrong and Jorgensen, 1994). A secondary process, called Ca2+-induced Ca2+ release (CICR), amplifies [Ca2+]i release in skeletal muscle, particularly under stress conditions in muscle fatigue and dystrophy (Fong et al., 1990; Takagi et al., 1992; Brotto et al., 2002).
Aging effects on muscle function have been associated with muscle fiber denervation, loss of motor units, and motor unit remodeling. Because functional alterations occur before significant muscle wasting becomes evident, changes in the E–C coupling machinery and [Ca2+]i homeostasis may act as causative factors for, or adaptive responses to, muscle aging (Larsson and Edstrom, 1986; Faulkner et al., 1995; Delbono, 2002). We show that stress-induced Ca2+ sparks, which are the elemental events of CICR in striated muscles (Cheng et al., 1993; Klein et al., 1996), are severely compromised in aged skeletal muscle. In addition, we find that muscle aging is associated with the development of a segregated SR Ca2+ pool that uncouples from the normal E–C coupling machinery. We present evidence to suggest that mitsugumin-29 (MG29) may act as a sentinel against the effects of age on skeletal muscle Ca2+ homeostasis.
Results and discussion
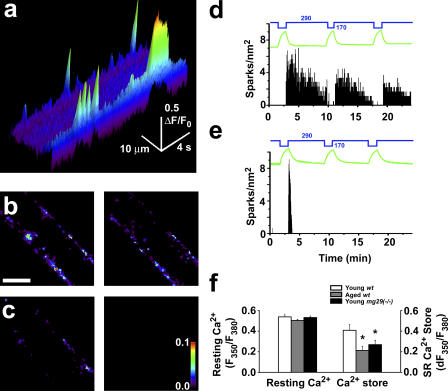
To delineate the contribution of Ca2+ sparks to the aging phenotype in skeletal muscle, intact flexor digitorum brevis (FDB) fibers isolated from young (2–4 mo) and aged (26–27 mo) mice were treated with osmotic shock to induce Ca2+ sparks. Exposure of the muscle fiber to a hypotonic solution leads to swelling of the fiber. Upon return to isotonic solution, the recovery of cell volume to normal is accompanied by a robust, peripherally localized Ca2+ spark response (Fig. 1 a; Wang et al., 2005). Young muscle fibers display a dynamic Ca2+ spark response to repeated stress cycles, with each round of osmotic shock generating Ca2+ sparks that continue for several minutes (Fig. 1, b and d). This Ca2+ spark response is located in the periphery of both young and aged muscle fibers. The dynamic nature of this Ca2+ spark response is significantly reduced in aged muscle (Fig. 1 c). Relative to young muscle (Fig. 1 d), aged muscle appears to contain a diminished capacity for the generation of dynamic Ca2+ sparks with repeated osmotic stresses (Fig. 1 e).
Figure 1.
Loss of plastic Ca2+ spark signaling in aged skeletal muscle. Intact FDB muscle fibers were treated with hypotonic shock to generate a Ca2+ spark response. (a) Cross section line scan image of young FDB muscle fiber after osmotic shock. Distinct Ca2+ spark and burst events are seen in the periphery of the muscle fiber. (b) Sparks over the span of 2 min were binned using a custom-designed IDL data processing software routine (left). Plasticity of Ca2+ sparks in young fibers is shown with the persistent response seen after a second osmotic shock on the same cell (right). Bar, 15 μm. (c) Aged skeletal muscle fibers show an initial response after osmotic shock (left); however, this response is blunted and cannot be observed in subsequent osmotic shocks (right). Pseudocolor represents the average number of Ca2+ release events at each point within the fiber, with a scale of 0.0–0.1 in sparks/s. (d) A diary plot of spark activity versus changes in fiber volume (green line) illustrates young fibers can produce three or more responses to osmotic shock (n = 16). Blue numbers and lines indicate the osmolarity of perfusion solutions. (e) Aged fibers only display a rapidly terminating response after the first shock, which cannot be restimulated (n = 14). (f) Determination of [Ca2+]i- (left) and caffeine/ryanodine-sensitive SR Ca2+ store (right) in FDB fibers as measured by changes in fura-2 fluorescence. Error bars are the mean ± SEM. * indicates P < 0.05 by analysis of variance (ANOVA).
The diminished Ca2+ spark response in aged muscle could result from changes in resting [Ca2+]i levels or altered Ca2+ storage inside the SR. We measured [Ca2+]i levels and SR Ca2+ storage in FDB muscle fibers obtained from young and aged mice. As shown in Fig. 1 f, the resting [Ca2+]i levels appear to be similar between young and aged skeletal muscle fibers, whereas the caffeine/ryanodine-mobilized SR Ca2+ pool is significantly less in aged skeletal muscle. Thus, the reduced caffeine/ryanodine-mobilized SR Ca2+ store may represent one potential factor for the compromised Ca2+ spark signaling associated with muscle aging.
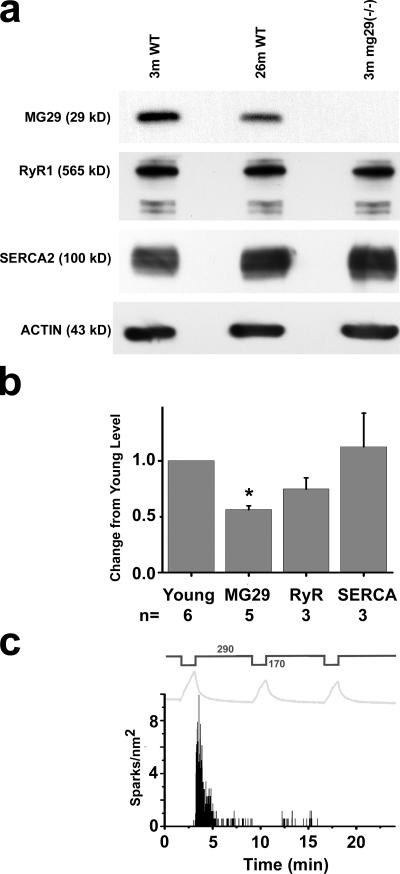
Additional factors that may contribute to this defective Ca2+ spark signaling include changes in membrane ultrastructure or altered expression of Ca2+ regulatory proteins in skeletal muscle. We conducted a survey of triad junction proteins and found that the expression level of MG29 (Takeshima et al., 1998), a synaptophysin-related membrane protein, is significantly down-regulated during muscle aging (Fig. 2, a and b). To determine the extent that decreased MG29 levels contribute to age-related alterations in muscle Ca2+ homeostasis, muscle fibers obtained from young (3–5 mo) mg29(−/−) mice (Nishi et al., 1999) were stressed by osmotic shock. As with aged wild-type (wt) muscle, there is an initial Ca2+ spark response to the first osmotic shock and subsequent osmotic shocks produce little to no Ca2+ spark response in young mg29(−/−) muscle fibers (Fig. 2 c). Using fura-2 Ca2+ measurements, we found that the resting [Ca2+]i level and SR Ca2+ storage are similar between aged wt and young mg29(−/−) muscle fibers (Fig. 1 f). These results point to a role for MG29 in maintaining normal Ca2+ homeostasis that is lost with its diminished expression during aging.
Figure 2.
Reduced expression of MG29 in aged skeletal muscle and compromised Ca2+ spark signaling in mg29(−/−) muscle. (a) Western blots were performed for MG29, RyR, and SERCA2 on FDB muscle extracts from young and aged wt mice, and from young mg29(−/−) mice. (b) Densitometry measurements of altered protein expression with muscle aging. Values are the mean change in protein level versus young wt (set at 100%) ± the SEM. n represents paired groups of young and aged mice. * represents P < 0.001. Levels of RyR1 appear to decrease in aged skeletal muscle, although these differences do not reach a level of statistical significance (P = 0.064). Variation in the level of RyR in aged muscle may result from the presence of tubular aggregates (Fig. 3). (c) Osmotic shock–induced Ca2+ sparks in mg29(−/−) muscle is plotted against changes in fiber volume (gray line) (n = 14).
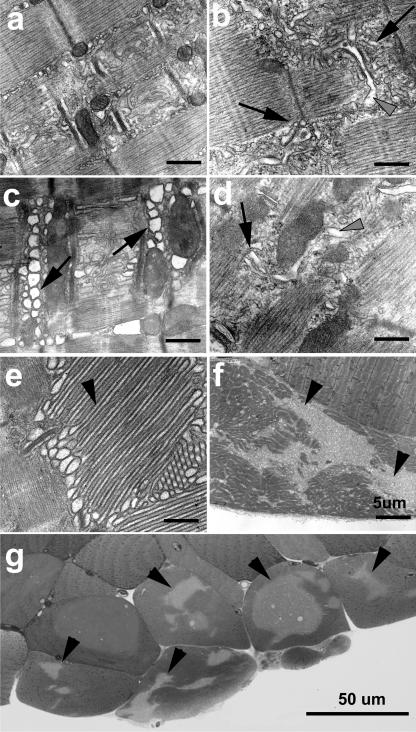
Our previous studies have shown that the mg29(−/−) mice display contractile alterations and muscle atrophy at ages of 6 mo or younger (Nishi et al., 1999; Nagaraj et al., 2000) that resemble the atrophic phenotype of aged wt mice. Electron microscopy studies reveal similar ultrastructural alterations to triad junction membrane structures of aged wt and young mg29(−/−) skeletal muscle. Although organized alignment of SR and transverse-tubule (TT) membranes is present in young wt muscle, fragmented SR is frequently observed in slow (soleus) and fast (extensor digitorum longus [EDL]) twitch muscles from both young mg29(−/−) and aged wt skeletal muscles (Fig. 3, b–d). Aged wt soleus muscle also displays swelling of the TT that is very similar to that seen in mg29(−/−) muscle (Nishi et al., 1999). The development of these defects appears to be progressive during aging, as EDL fibers display a continuum of damage ranging from minor SR fragmentation (Fig. 3 c) to formation of large aggregations of SR (Fig. 3 e and Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200604166/DC1; Agbulut et al., 2000; Chevessier et al., 2004). We suspect that aggregation of SR may result from the accumulation of subtle defects in Ca2+ signaling and membrane recycling, leading to progressive sarcopenia (Fig. 3, f and g).
Figure 3.
Disruption of triad junction membrane structures in aged wild-type and young mg29(−/−) mice. EDL from young wt (a) and hindlimb muscle from young mg29(−/−) animals (b), as well as aged wt EDL (c) and soleus (d), were examined by transmission electron microscopy. Many cells from aged EDL display large aggregates of SR (e) that are preferentially found in necrotic cells (f). Light microscopy of a toluidine blue–stained transverse section reveals the necrotic nature of aged muscle fibers containing large SR aggregates (g). Arrows indicate fragmented SR network; black arrowheads indicate tubular aggregates; gray arrowheads point to swollen TT. The presence of intact mitochondria minimizes the risk of fixation artifact in these micrographs. Bars, 0.5 μm, unless otherwise noted.
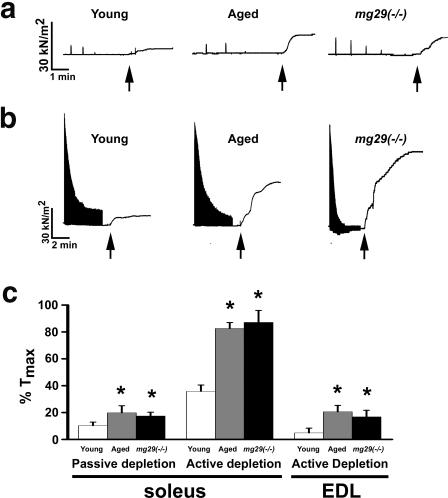
The similar structural alterations seen in aged wt and young mg29(−/−) muscle suggests that further defects in Ca2+ homeostasis beyond their Ca2+ spark response should be present. Previous studies by Kurebayashi et al. (2003) revealed that repetitive Ca2+ release using KCl stimulation on intact mg29(−/−) muscle in the absence of extracellular Ca2+ ([Ca2+]o) did not lead to complete depletion of the SR Ca2+ pool. We now present evidence to show the presence of a segregated SR Ca2+ pool that uncouples from the normal voltage-induced Ca2+ release (VICR) machinery in young mg29(−/−) muscle and aged wt muscle (Fig. 4). When isolated, intact muscle fibers are exposed to 0 [Ca2+]o for 90 min, intermittent nonfatigue voltage stimulation leads to passive depletion of the SR Ca2+ pool, followed by minimal caffeine-induced Ca2+ release in young wt muscle, whereas this response is larger in aged wt and young mg29(−/−) muscle (Fig. 4 a). In the absence of [Ca2+]o, fatiguing stimulation using repetitive VICR leads to rapid depletion of the voltage-sensitive pool of [Ca2+]i releasable, revealing striking differences among the three muscle preparations in their subsequent response to caffeine treatment. As shown in Fig. 4 b, after this fatiguing stimulation, caffeine induces significantly larger Ca2+ release in young mg29(−/−) and aged wt muscles compared with the young wt muscle. Data from multiple experiments are summarized in Fig. 4 c. These findings suggest the presence of a VICR-uncoupled, RyR-sensitive Ca2+ pool in aged wt and young mg29(−/−) muscles; however, the relative sizes of these pools in soleus and EDL muscle fibers are difficult to evaluate because of increased sensitivity of the contractile apparatus of the soleus muscle to caffeine and Ca2+ (Singh and Dryden, 1989; Lamb et al., 2001; Jin et al., 2003; Brotto et al., 2006).
Figure 4.
Segregation of [Ca2+]i release in aged wild-type and young mg29(−/−) muscle. (a) Force tracings of soleus muscle bundles after passive depletion of [Ca2+]i stores by single tetanic voltage stimulation contractions once per minute during incubation in 0 [Ca2+]o for ∼90 min. Traces are cropped to only include the final six minutes of the experiment. 30 mM caffeine was added at points indicated by the arrows to induce contraction after the force generated by voltage stimulation could no longer be detected. (b) Tetanic voltage stimulation, for a 5-min duration, was applied to isolated soleus muscles in a solution containing 0 [Ca2+]o. Arrows indicate the application of 30 mM caffeine. Although young wt muscles show a minimal response to caffeine, young mg29(−/−) and aged wt muscles display a significantly enhanced response to caffeine, indicating the presence of a Ca2+ pool that cannot be mobilized by extended voltage stimulation. (c) Data from multiple experiments are averaged (mean ± SEM; n = 6–11). Data is provided for both soleus and EDL skeletal muscles. * indicates significant differences, with P < 0.05 as measured by ANOVA.
The segregated SR Ca2+ pool in aged wt and young mg29(−/−) muscle is not likely to result from major changes in the VICR machinery itself, as the initial VICR responses in young and aged wt and young mg29(−/−) muscle are generally similar (Fig. 4, a and b). The maximal specific tetanic force produced by aged wt EDL muscle (250 ± 15 kN/m2) is lower than that produced by young wt muscle (300 ± 18 kN/m2), which is consistent with previous studies (Faulkner et al., 1995; Gonzalez et al., 2000). The maximal specific tetanic force in young mg29(−/−) EDL muscles is 262 ± 17 kN/m2, which is similar to that measured in aged wt muscle. To test the possibility that changes in contractile machinery during muscle aging might contribute to our measurement of Ca2+ release, the force versus pCa relationship was examined in all three types of muscle preparations. As shown in Table S2 (available at http://www.jcb.org/cgi/content/full/jcb.200604166/DC1), the isometric contractile properties of Triton X-100–skinned muscle fibers are similar in young and aged wt, as well as young mg29(−/−), mice. Therefore, under these conditions, the force output is an authentic measurement of Ca2+ release from the SR, and a segregated SR Ca2+ pool must exist in aged wt and young mg29(−/−) muscle to account for the elevated, caffeine-induced Ca2+ release.
We have established that aged muscle fibers have a disrupted Ca2+ spark response and a segregated Ca2+ store that cannot be mobilized by VICR, which are associated with ultrastructural disruption of triad junctions and the SR network (Fig. 5). One possible explanation for the development of a segregated SR Ca2+ pool is that subtle disruption of SR and TT alignment at the triad junction could result in uncoupling of RyR1 and DHPR, which could also lead to the compromised Ca2+ spark signaling observed in aged skeletal muscle. An inhibitory role for DHPR on RyR1 function has been proposed by other investigators (Suda and Penner, 1994; Lee et al., 2004; Zhou et al., 2006). If the Ca2+ spark response associated with membrane deformation and the segregation of [Ca2+]i release was solely caused by disruption of the inhibitory effects of DHPR on RyR1 function, one would expect to see an elevated Ca2+ spark response in aged skeletal muscle, as it has been established that DHPR expression and the ratio of DHPR to RyR1 is decreased in aged skeletal muscle (Renganathan et al., 1997). Therefore, the reduced Ca2+ spark response observed in aged skeletal muscle suggests that changes in other cellular factors, such as MG29 expression, may play a role in regulation of Ca2+ signaling in skeletal muscle at different developmental stages.
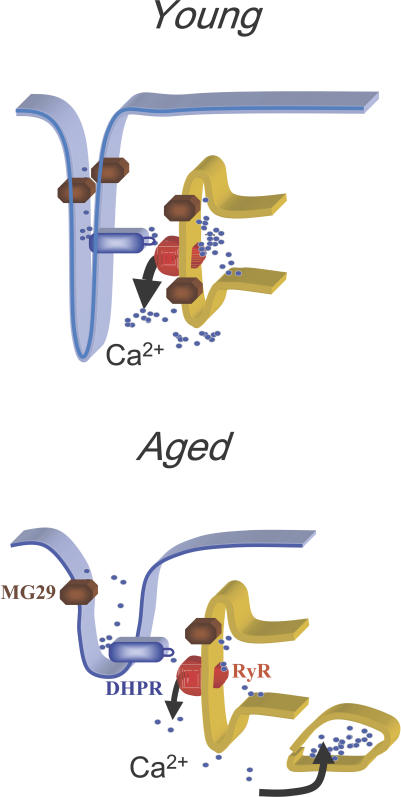
Figure 5.
Schematic diagram to illustrate the segregated Ca2+ release process in aged skeletal muscle. In young, healthy skeletal muscle, plastic activation of Ca2+ sparks represents a physiologic response to stress. This process is reduced in aged muscle because of the following two factors: first, that reduced expression of MG29 may lead to an increased threshold for Ca2+-induced activation of RyR; and second, that fragmentation of SR enables generation of a segregated SR Ca2+ pool that uncouples from the normal VICR process.
MG29 contains a high degree of homology with synaptophysin, a protein thought to be involved with membrane fusion during exocytosis (Alder et al., 1992). Decreased MG29 expression may lead to improper lipid membrane formation or fusion, altering the dynamic process of membrane recycling and SR network formation. In aging wt or young mg29(−/−) mice, the lack of this synaptophysin family member would suppress the efficient maintenance of triad junction structure, while also generating a fragmented SR network. Recent results from our laboratory have begun to shed light on the physiological function of MG29. We have demonstrated that MG29 increases sensitivity of the RyR channel to CICR when expressed in a heterologous cell system and when reconstituted with RyR in lipid bilayer single-channel studies (Pan et al., 2004). The lack of MG29 in aged and mg29(−/−) muscle should decrease the sensitivity of RyR to CICR, in agreement with our current results.
Segregation of Ca2+ pools in aged muscle may have a physiological role in maintaining muscle integrity in the face of decreasing homeostatic capabilities. The resulting dampened Ca2+ mobilization in aged muscle may be a compensatory mechanism that protects aged fibers from Ca2+-induced injury. It is also possible that the presence of a segregated Ca2+ reserve isolated from VICR responses contributes to cellular stress and decreased homeostatic capacity. Although the mechanism of active shuttling of Ca2+ from the VICR-responsive to the VICR-nonresponsive pool is not known, selective regulation of this Ca2+ shuttling to modulate the VICR-responsive pool would allow for the enhancement of aging skeletal muscle performance and/or protect skeletal muscle during aging. The mg29(−/−) mouse represents a model system in which these mechanisms can be examined and these hypotheses can be tested.
Materials and methods
Ca2+ imaging and spark analysis
Studies were conducted with wt C57Bl6/J male mice (Aged Rodent Colony, maintained by the National Institute on Aging), which were either 2–4 or 26–27 mo old. Male mg29(−/−) mice (2–5 mo old), and wt animals of the same genetic background (129Sv/J backcrossed to C57Bl6/J) were maintained in local facilities and handled in a manner approved by local regulations. FDB fibers were isolated by enzymatic disassociation in 0.2% type I collagenase (Sigma-Aldrich) for 55 min at 37°C and loaded with 10 μM Fluo-4–AM for 60 min at room temperature. Mean FDB fiber size was 1 mm × 20 μM. Measurements of Ca2+ release were performed on a confocal microscope (Radiance 2100; Bio-Rad Laboratories) equipped with an argon laser (488 nm) and a 60×, 1.3 NA, oil immersion objective. For Ca2+ spark measurements, fibers were perfused with a 170-mosM hypotonic solution containing (in mM) 70 NaCl, 5 KCl, 10 Hepes, 2.5 CaCl2, 2 MgCl2, pH 7.2, for 60–180 s to induce swelling before perfusion was switched back to the initial Tyrode solution (in mM) 140 NaCl, 5 KCl, 10 Hepes, 2.5 CaCl2, 2 MgCl2, pH 7.2, with an osmolarity of 290 mosM as measured by a Micro Osmometer 3300 (Advanced Instruments). Image analysis was performed using custom routines on IDL software (Research Systems, Inc.; Cheng et al., 1999; Wang et al., 2005).
For determination of resting cytosolic Ca2+ levels and total SR Ca2+ store, individual FDB fibers were loaded with 10 μM fura-2 AM for 45 min at room temperature in Tyrode solution. 20 μM N-benzyl-p-toluene sulphonamide, a myosin II inhibitor, was applied for 15 min to prevent motion artifact from muscle contraction (Cheung et al., 2002; Pinniger et al., 2005). Fibers were also embedded into silicone grease to maintain their position in the culture dish (Jacquemond, 1997). The ratio of fura-2 fluorescence at excitation wavelength of 350 and 380 nm was measured on a PTI spectrofluorometer (Photon Technology International) to assess the resting [Ca2+]i level. The SR Ca2+ store was measured by addition of 20 mM caffeine plus 5 μM ryanodine in the presence of 0 [Ca2+]o.
Electron and light microscopy
Electron microscopy studies were performed following our previously published protocols (Ito et al., 2001). In brief, skeletal muscles were fixed in 3% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M cacodylate buffer, pH 7.4, and later postfixed in 1% OsO4 and 0.1 M cacodylate buffer, pH 7.4. Microthin sections were double stained with uranyl acetate and lead citrate. These sections were examined under a transmission electron microscope (JEM-1010; JEOL).
Intact muscle preparation
Intact EDL and soleus muscles were dissected from mice and maintained in modified Ringer's solution containing the following (in mM): 142 NaCl, 4.0 KCl, 2.5 CaCl2, 2.0 MgCl2, 10 glucose, and 10 Hepes, pH 7.4 ± 0.1, continuously bubbled with 100% O2. EDL muscles had a mean length of 12 mm and a mean mass of 80 mg, whereas soleus muscles had a mean length of 10 mm and a mean mass of 10 mg. Muscles were mounted vertically on a glass-stimulating apparatus (Radnoti) with platinum electrodes and attached to a movable isometric force transducer and to a stationary anchor, which allowed muscles to be stretched until both maximal forces for a given frequency and the frequency producing Tmax were obtained.
Force measurements during passive depletion and fatigue
After Tmax was determined, the intact muscles were allowed to equilibrate for 20 min in the Ringer's solution. During equilibration, muscle strips were stimulated with ∼100–120 Hz (EDL) or ∼60–80 Hz (SOL), 330 mA, 500 ms electrical pulse–trains administered with a periodicity of 1 min to generate Tmax. After equilibration, the muscles from the passive-depletion group were washed five times in Ringer's solution with the same composition as described in the previous section, except that no CaCl2 was added while 0.1 mM EGTA was added, to create a nominal 0 [Ca2+]o solution. Muscles were stimulated with one Tmax every minute in 0 [Ca2+]o solution until force declined to nondetectable levels; the muscles were then exposed to 30 mM caffeine. Force produced in response to caffeine application was recorded until a stable plateau was obtained. After the passive depletion protocol, muscles were subjected to extensive washes in normal Ringer's solution containing 2.5 Ca2+ and then electrically stimulated until force returned to initial equilibration values. In 80% of our preparations, this was achieved. After forces were stable and comparable to the initial levels before the onset of the passive depletion, muscles were returned to the 0 [Ca2+]o solution for 5 min and subsequently subjected to a 1–5 min fatiguing protocol consisting of the same stimulatory pattern administered at a 1-s periodicity (i.e., 50% duty cycle). In between fatigue runs, muscles were washed in 2.5 Ca2+ solution and force was allowed to recover to prefatigue levels before the onset of the next fatigue run. At the end of each fatiguing protocol, muscles were treated with 30 mM caffeine and maximal response to caffeine was recorded. Caffeine was mixed in a small volume of the Ringer's solution and added to the chambers to produce a final concentration of 30 mM in the bathing chamber. Whenever possible, paired experiments were performed with young wild-type animals and aged wt or young mg29(−/−) animals. Experiments were also conducted with fibers only exposed to passive depletion or fatigue in 0 [Ca2+]o to confirm that effects of each treatment can be observed independently. The integrity of the fiber contractile apparatus and Ca2+-handling machinery was tested at the conclusion of the protocol by exposure to 100 mM KCl. Only fibers with at least 85% of Tmax were included for statistical analysis. All force data were normalized to the last tetanic contraction at the end of the equilibration period and just before the start of the fatiguing protocol (this Tmax = 100%). Absolute force, normalized per cross sectional area (i.e., in kN/m2) was determined at the end of the equilibration period by the following relationship: Force (in Kg) = (g of force) × (muscle length in cm) × 1.06/muscle weight (g), where 1.06 represents the density of the muscle strips. Triton X-100–skinned muscle fiber experiments followed the protocols as previously described (Brotto and Nosek, 1996; Brotto et al., 2004).
Statistical analysis
All statistical analysis in this study was conducted using ANOVA, and data is presented as the mean ± SEM.
Online supplemental material
Table S1 describes the parallel disruption of triad junctions in aged wt and young mg29(−/−) skeletal muscle. Table S2 is an assessment of muscle contractile function in young wt, aged wt, and young mg29(−/−) muscle. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200604166/DC1.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants (RO1-AG15556, RO1-HL69000, RO1-CA95739, and RO1-AG28614) to J. Ma, a NIH grant to J. Parness (RO1-AR45593), a National Institute on Aging Faculty Development grant to M. Brotto, and an American Heart Association postdoctoral fellowship to N. Weisleder.
N. Weisleder and M. Brotto contributed equally to this paper.
Abbreviations used in this paper: ANOVA, analysis of variance; CICR, Ca2+-induced Ca2+ release; E–C, excitation–contraction; EDL, extensor digitorum longus; [Ca2+]o, extracellular Ca2+; FDB, flexor digitorum brevis; [Ca2+]i, intracellular Ca2+; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; TT, transverse tubule; VICR, voltage-induced Ca2+ release; wt, wild-type.
References
- Agbulut, O., J. Destombes, D. Thiesson, and G. Butler-Browne. 2000. Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem. Cell Biol. 114:477–481. [DOI] [PubMed] [Google Scholar]
- Alder, J., Z.P. Xie, F. Valtorta, P. Greengard, and M. Poo. 1992. Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron. 9:759–768. [DOI] [PubMed] [Google Scholar]
- Brotto, M.A., and T.M. Nosek. 1996. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J. Appl. Physiol. 81:731–737. [DOI] [PubMed] [Google Scholar]
- Brotto, M.A., T.M. Nosek, and R.C. Kolbeck. 2002. Influence of ageing on the fatigability of isolated mouse skeletal muscles from mature and aged mice. Exp. Physiol. 87:77–82. [DOI] [PubMed] [Google Scholar]
- Brotto, M.A., R.Y. Nagaraj, L.S. Brotto, H. Takeshima, J.J. Ma, and T.M. Nosek. 2004. Defective maintenance of intracellular Ca2+ homeostasis is linked to increased muscle fatigability in the MG29 null mice. Cell Res. 14:373–378. [DOI] [PubMed] [Google Scholar]
- Brotto, M.A., B.J. Biesiadecki, L.S. Brotto, T.M. Nosek, and J.P. Jin. 2006. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am. J. Physiol. Cell Physiol. 290:C567–C576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H., W.J. Lederer, and M.B. Cannell. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. [DOI] [PubMed] [Google Scholar]
- Cheng, H., L.S. Song, N. Shirokova, A. Gonzalez, E.G. Lakatta, E. Rios, and M.D. Stern. 1999. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys. J. 76:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, A., J.A. Dantzig, S. Hollingworth, S.M. Baylor, Y.E. Goldman, T.J. Mitchison, and A.F. Straight. 2002. A small-molecule inhibitor of skeletal muscle myosin II. Nat. Cell Biol. 4:83–88. [DOI] [PubMed] [Google Scholar]
- Chevessier, F., I. Marty, M. Paturneau-Jouas, D. Hantai, and M. Verdiere-Sahuque. 2004. Tubular aggregates are from whole sarcoplasmic reticulum origin: alterations in calcium binding protein expression in mouse skeletal muscle during aging. Neuromuscul. Disord. 14:208–216. [DOI] [PubMed] [Google Scholar]
- Delbono, O. 2002. Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle. Biogerontology. 3:265–270. [DOI] [PubMed] [Google Scholar]
- Faulkner, J.A., S.V. Brooks, and E. Zerba. 1995. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J. Gerontol. A Biol. Sci. Med. Sci. 50 Spec No:124–129. [DOI] [PubMed] [Google Scholar]
- Fong, P.Y., P.R. Turner, W.F. Denetclaw, and R.A. Steinhardt. 1990. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 250:673–676. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., and A.O. Jorgensen. 1994. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 56:509–534. [DOI] [PubMed] [Google Scholar]
- Gonzalez, E., M.L. Messi, and O. Delbono. 2000. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J. Membr. Biol. 178:175–183. [DOI] [PubMed] [Google Scholar]
- Ito, K., S. Komazaki, K. Sasamoto, M. Yoshida, M. Nishi, K. Kitamura, and H. Takeshima. 2001. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol. 154:1059– 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond, V. 1997. Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophys. J. 73:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J.P., M.A. Brotto, M.M. Hossain, Q.Q. Huang, L.S. Brotto, T.M. Nosek, D.H. Morton, and T.O. Crawford. 2003. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J. Biol. Chem. 278:26159–26165. [DOI] [PubMed] [Google Scholar]
- Klein, M.G., H. Cheng, L.F. Santana, Y.H. Jiang, W.J. Lederer, and M.F. Schneider. 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 379:455–458. [DOI] [PubMed] [Google Scholar]
- Kurebayashi, N., H. Takeshima, M. Nishi, T. Murayama, E. Suzuki, and Y. Ogawa. 2003. Changes in Ca2+ handling in adult MG29-deficient skeletal muscle. Biochem. Biophys. Res. Commun. 310:1266–1272. [DOI] [PubMed] [Google Scholar]
- Lamb, G.D., M.A. Cellini, and D.G. Stephenson. 2001. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J. Physiol. 531:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, L., and L. Edstrom. 1986. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J. Neurol. Sci. 76:69–89. [DOI] [PubMed] [Google Scholar]
- Lee, E.H., J.R. Lopez, J. Li, F. Protasi, I.N. Pessah, H. Kim do, and P.D. Allen. 2004. Conformational coupling of DHPR and RyR1 in skeletal myotubes is influenced by long-range allosterism: evidence for a negative regulatory module. Am. J. Physiol. Cell Physiol. 286:C179–C189. [DOI] [PubMed] [Google Scholar]
- Ma, J., M. Fill, C.M. Knudson, K.P. Campbell, and R. Coronado. 1988. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 242:99–102. [DOI] [PubMed] [Google Scholar]
- Nagaraj, R.Y., C.M. Nosek, M.A. Brotto, M. Nishi, H. Takeshima, T.M. Nosek, and J. Ma. 2000. Increased susceptibility to fatigue of slow- and fast-twitch muscles from mice lacking the MG29 gene. Physiol. Genomics. 4:43–49. [DOI] [PubMed] [Google Scholar]
- Nishi, M., S. Komazaki, N. Kurebayashi, Y. Ogawa, T. Noda, M. Iino, and H. Takeshima. 1999. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J. Cell Biol. 147:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z., Y. Hirata, R.Y. Nagaraj, J. Zhao, M. Nishi, S.M. Hayek, M.B. Bhat, H. Takeshima, and J. Ma. 2004. Co-expression of MG29 and ryanodine receptor leads to apoptotic cell death: effect mediated by intracellular Ca2+ release. J. Biol. Chem. 279:19387–19390. [DOI] [PubMed] [Google Scholar]
- Pinniger, G.J., J.D. Bruton, H. Westerblad, and K.W. Ranatunga. 2005. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J. Muscle Res. Cell Motil. 26:135–141. [DOI] [PubMed] [Google Scholar]
- Renganathan, M., M.L. Messi, and O. Delbono. 1997. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 157:247–253. [DOI] [PubMed] [Google Scholar]
- Rios, E., G. Pizarro, and E. Stefani. 1992. Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu. Rev. Physiol. 54:109–133. [DOI] [PubMed] [Google Scholar]
- Singh, Y.N., and W.F. Dryden. 1989. Isometric contractile properties and caffeine sensitivity of the diaphragm, EDL and soleus in the mouse. Clin. Exp. Pharmacol. Physiol. 16:581–589. [DOI] [PubMed] [Google Scholar]
- Suda, N., and R. Penner. 1994. Membrane repolarization stops caffeine-induced Ca2+ release in skeletal muscle cells. Proc. Natl. Acad. Sci. USA. 91:5725–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi, A., S. Kojima, M. Ida, and M. Araki. 1992. Increased leakage of calcium ion from the sarcoplasmic reticulum of the mdx mouse. J. Neurol. Sci. 110:160–164. [DOI] [PubMed] [Google Scholar]
- Takeshima, H., M. Shimuta, S. Komazaki, K. Ohmi, M. Nishi, M. Iino, A. Miyata, and K. Kangawa. 1998. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 331:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., N. Weisleder, C. Collet, J. Zhou, Y. Chu, Y. Hirata, X. Zhao, Z. Pan, M. Brotto, H. Cheng, and J. Ma. 2005. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7:525–530. [DOI] [PubMed] [Google Scholar]
- Zhou, J., J. Yi, L. Royer, B.S. Launikonis, A. Gonzalez, J. Garcia, and E. Rios. 2006. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 290:C539–C553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.