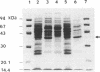
Abstract
Using Bacillus subtilis as a host and pTB524 as a vector plasmid, we cloned the thermostable alcohol dehydrogenase (ADH-T) gene (adhT) from Bacillus stearothermophilus NCA1503 and determined its nucleotide sequence. The deduced amino acid sequence (337 amino acids) was compared with the sequences of ADHs from four different origins. The amino acid residues responsible for the catalytic activity of horse liver ADH had been clarified on the basis of three-dimensional structure. Since those catalytic amino acid residues were fairly conserved in ADH-T and other ADHs, ADH-T was inferred to have basically the same proton release system as horse liver ADH. The putative proton release system of ADH-T was elucidated by introducing point mutations at the catalytic amino acid residues, Cys-38 (cysteine at position 38), Thr-40, and His-43, with site-directed mutagenesis. The mutant enzyme Thr-40-Ser (Thr-40 was replaced by serine) showed a little lower level of activity than wild-type ADH-T did. The result indicates that the OH group of serine instead of threonine can also be used for the catalytic activity. To change the pKa value of the putative system, His-43 was replaced by the more basic amino acid arginine. As a result, the optimum pH of the mutant enzyme His-43-Arg was shifted from 7.8 (wild-type enzyme) to 9.0. His-43-Arg exhibited a higher level of activity than wild-type enzyme at the optimum pH.
Full text
PDF
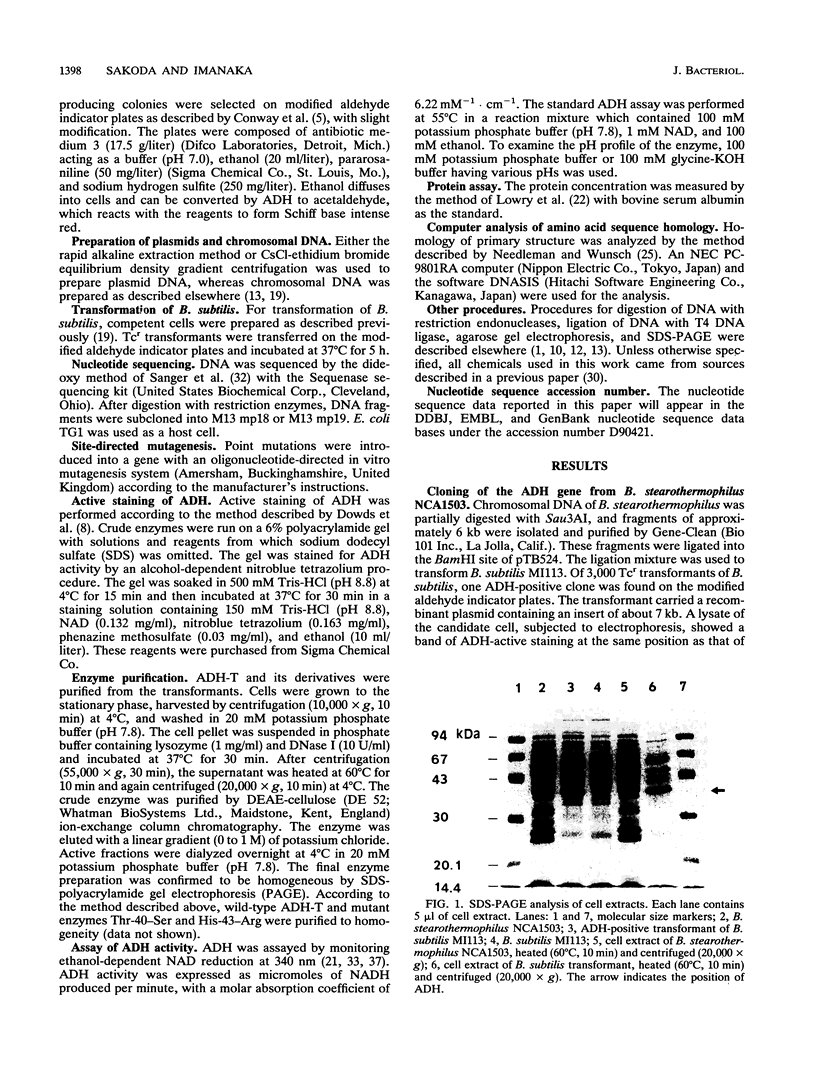


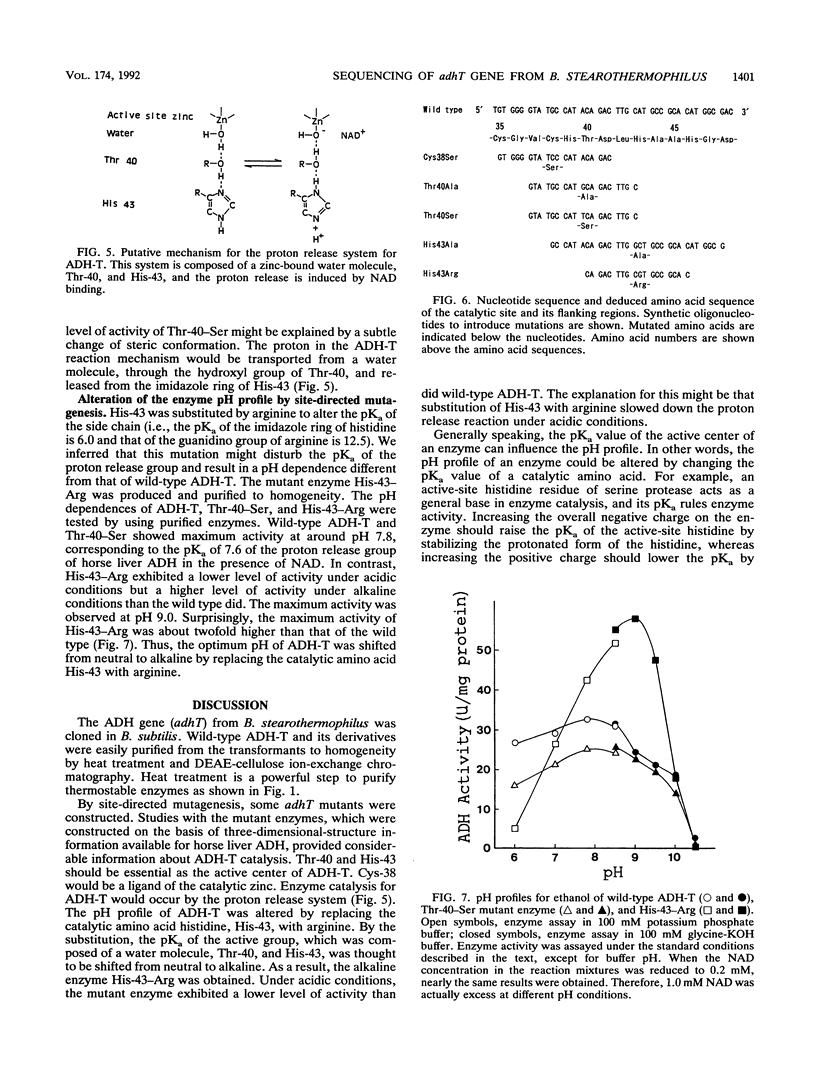

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Kitai K., Imanaka T. Cloning and Expression of Thermostable alpha-Amylase Gene from Bacillus stearothermophilus in Bacillus stearothermophilus and Bacillus subtilis. Appl Environ Microbiol. 1983 Nov;46(5):1059–1065. doi: 10.1128/aem.46.5.1059-1065.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A., Ellwood D. C., Evans C. G., Yeo R. G. Production of alcohol by Bacillus stearothermophilus. Biotechnol Bioeng. 1975 Sep;17(9):1375–1377. doi: 10.1002/bit.260170914. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Kolb E., Harris J. I. Amino acid sequence homology in alcohol dehydrogenase. FEBS Lett. 1973 Jun 15;33(1):1–3. doi: 10.1016/0014-5793(73)80144-9. [DOI] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds B. C., Sheehan M. C., Bailey C. J., McConnell D. J. Cloning and characterization of the gene for a methanol-utilising alcohol dehydrogenase from Bacillus stearothermophilus. Gene. 1988 Aug 15;68(1):11–22. doi: 10.1016/0378-1119(88)90594-x. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Brändén C. I. The structure of horse liver alcohol dehydrogenase. FEBS Lett. 1974 Aug 25;44(2):200–204. doi: 10.1016/0014-5793(74)80725-8. [DOI] [PubMed] [Google Scholar]
- Fujii M., Takagi M., Imanaka T., Aiba S. Molecular cloning of a thermostable neutral protease gene from Bacillus stearothermophilus in a vector plasmid and its expression in Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1983 May;154(2):831–837. doi: 10.1128/jb.154.2.831-837.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T., Fujiyoshi T., Kurachi K., Yoshida A. Molecular cloning of a full-length cDNA for human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1985 May;82(9):2703–2707. doi: 10.1073/pnas.82.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aiba S. Isolation and characterization of antibiotic resistance plasmids from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981 Jun;146(3):1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aramori I., Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Himeno T., Aiba S. Effect of in vitro DNA rearrangement in the NH2-terminal region of the penicillinase gene from Bacillus licheniformis on the mode of expression in Bacillus subtilis. J Gen Microbiol. 1985 Jul;131(7):1753–1763. doi: 10.1099/00221287-131-7-1753. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Shibazaki M., Takagi M. A new way of enhancing the thermostability of proteases. Nature. 1986 Dec 18;324(6098):695–697. doi: 10.1038/324695a0. [DOI] [PubMed] [Google Scholar]
- Jeck R., Woenckhaus C., Harris J. J., Runswick M. J. Identification of the amino acid residue modified in Bacillus stearothermophilus alcohol dehydrogenase by the NAD+ analogue 4-(3-bromoacetylpyridinio)butyldiphosphoadenosine. Eur J Biochem. 1979 Jan 2;93(1):57–64. doi: 10.1111/j.1432-1033.1979.tb12794.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Sep;16(1):25–40. doi: 10.1111/j.1432-1033.1970.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Kuriki T., Okada S., Imanaka T. New type of pullulanase from Bacillus stearothermophilus and molecular cloning and expression of the gene in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1554–1559. doi: 10.1128/jb.170.4.1554-1559.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Imanaka T. Expression of the insecticidal protein gene from Bacillus thuringiensis subsp. aizawai in Bacillus subtilis and in the thermophile Bacillus stearothermophilus by using the alpha-amylase promoter of the thermophile. Appl Environ Microbiol. 1989 Dec;55(12):3208–3213. doi: 10.1128/aem.55.12.3208-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Rothstein D. M. Clostridium thermosaccharolyticum strain deficient in acetate production. J Bacteriol. 1986 Jan;165(1):319–320. doi: 10.1128/jb.165.1.319-320.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick M. J., Harris J. I. Purification of alcohol dehydrogenase from Bacillus stearothermophilus by affinity chromatography. FEBS Lett. 1978 Aug 15;92(2):365–367. doi: 10.1016/0014-5793(78)80788-1. [DOI] [PubMed] [Google Scholar]
- Russell A. J., Fersht A. R. Rational modification of enzyme catalysis by engineering surface charge. Nature. 1987 Aug 6;328(6130):496–500. doi: 10.1038/328496a0. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan M. C., Bailey C. J., Dowds B. C., McConnell D. J. A new alcohol dehydrogenase, reactive towards methanol, from Bacillus stearothermophilus. Biochem J. 1988 Jun 15;252(3):661–666. doi: 10.1042/bj2520661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Takada H., Imanaka T. Nucleotide sequence and cloning in Bacillus subtilis of the Bacillus stearothermophilus pleiotropic regulatory gene degT. J Bacteriol. 1990 Jan;172(1):411–418. doi: 10.1128/jb.172.1.411-418.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. recE4-Independent recombination between homologous deoxyribonucleic acid segments of Bacillus subtilis plasmids. J Bacteriol. 1979 Sep;139(3):775–782. doi: 10.1128/jb.139.3.775-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S., Theorell H., Akeson A. Dissociation constants of the binary complex of homogeneous horse liver alcohol dehydrogenase and nicotiniumamide adenine dinucleotide. Acta Chem Scand. 1967;21(7):1903–1920. doi: 10.3891/acta.chem.scand.21-1903. [DOI] [PubMed] [Google Scholar]
- Wills C. Production of yeast alcohol dehydrogenase isoenzymes by selection. Nature. 1976 May 6;261(5555):26–29. doi: 10.1038/261026a0. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]