Abstract
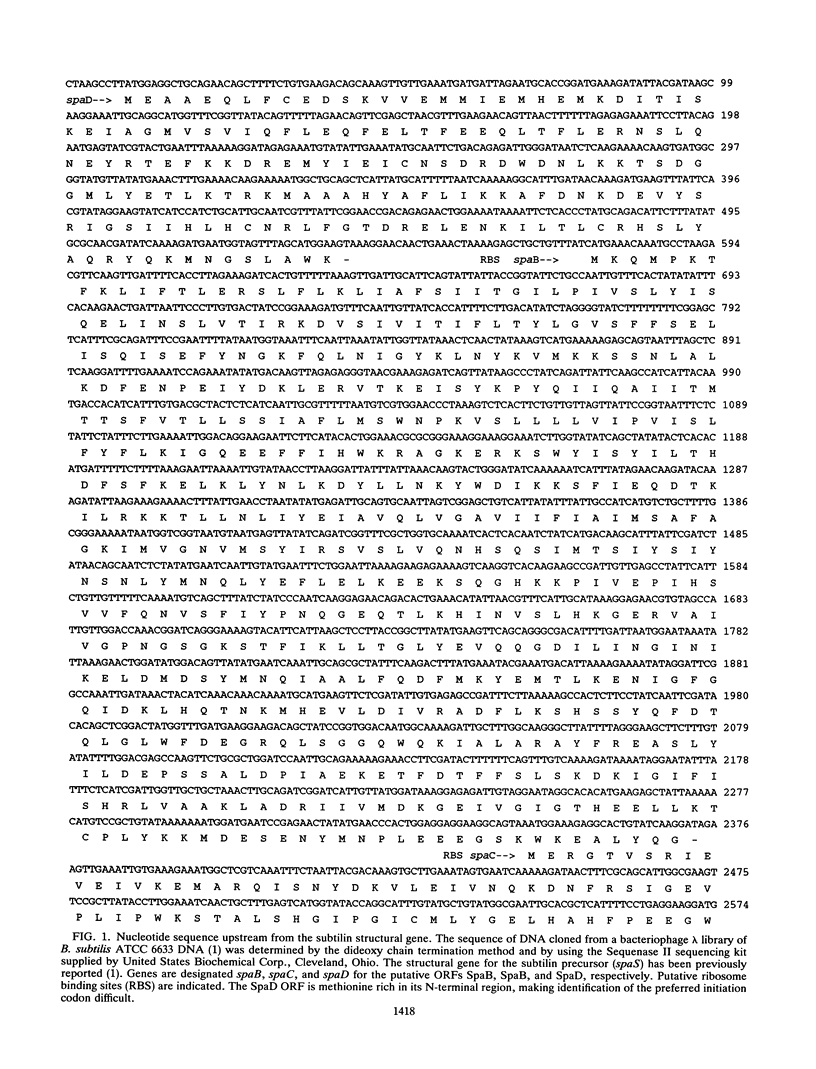
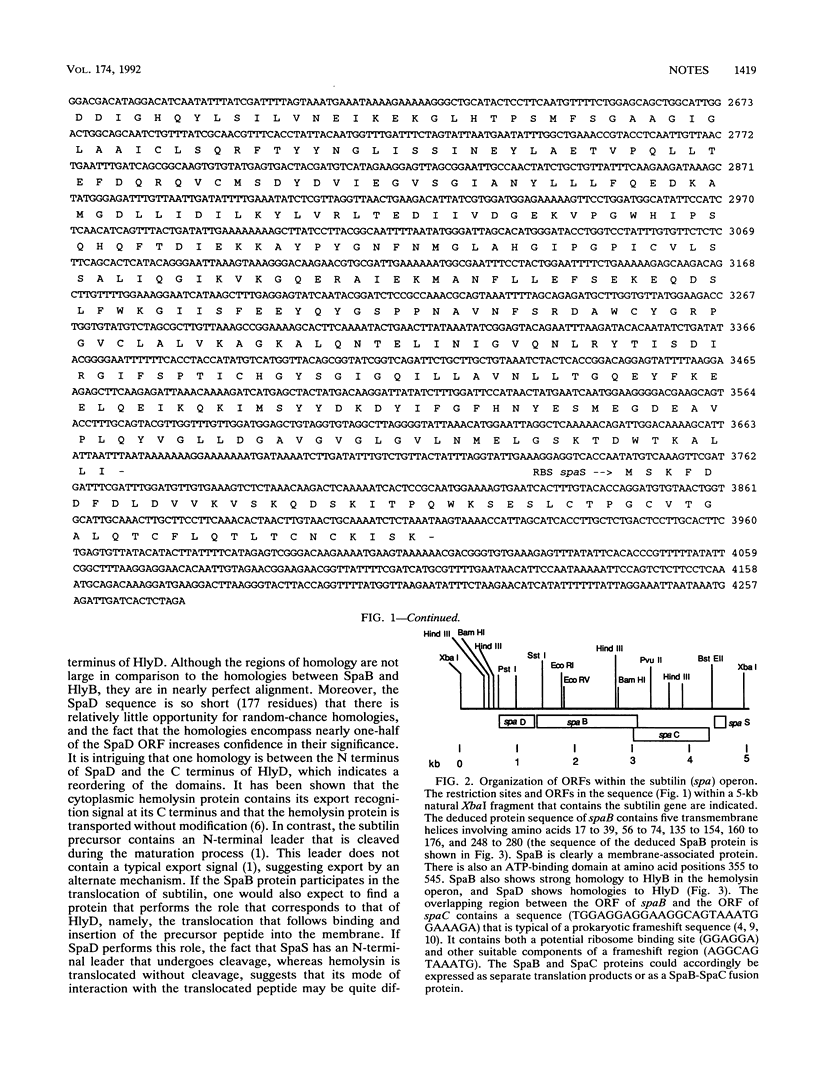
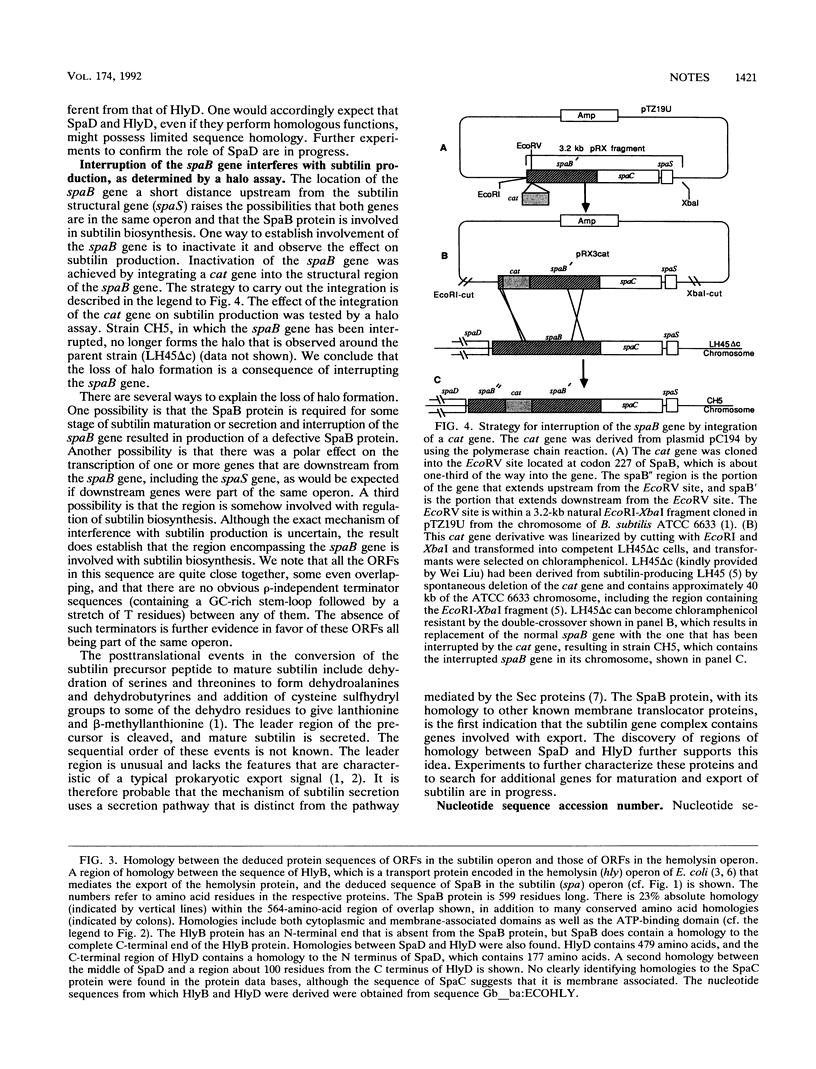
Sequence analysis upstream from the subtilin structural gene (spaS) in Bacillus subtilis ATCC 6633 revealed several open reading frames, SpaB, SpaC, and SpaD. SpaB, consisting of 599 amino acid residues, shows excellent homology with a variety of membrane translocator proteins, such as HlyB from Escherichia coli and some mammalian multidrug resistance proteins. When the spaB gene was interrupted by integration of a chloramphenicol acetyltransferase gene, the ability of the cell to produce subtilin, as determined by a halo assay, was lost. The homology of SpaB to translocator proteins, including transmembrane and ATP-binding regions, suggests that SpaB may play a role in subtilin secretion. The SpaB open reading frame overlaps with another open reading frame called SpaC, and the possibility that the SpaB and SpaC proteins become fused by frameshifting is considered. Regions of homology between SpaD (177 residues) and HlyD were also found, suggesting that SpaD may participate with SpaB in translocation of subtilin through the membrane. Although no readily interpretable homologies to SpaC (442 residues) were found, its sequence suggests that it is membrane associated. The absence of rho-independent transcription terminators between these open reading frames suggests that they are all part of the same operon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Hansen J. N. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem. 1988 Jul 5;263(19):9508–9514. [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Holland I. B., Kenny B., Blight M. Haemolysin secretion from E coli. Biochimie. 1990 Feb-Mar;72(2-3):131–141. doi: 10.1016/0300-9084(90)90138-7. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Conversion of Bacillus subtilis 168 to a subtilin producer by competence transformation. J Bacteriol. 1991 Nov;173(22):7387–7390. doi: 10.1128/jb.173.22.7387-7390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Speth W., Imhof B., Gentschev I., Goebel W. Translocation and compartmentalization of Escherichia coli hemolysin (HlyA). J Bacteriol. 1990 Jul;172(7):3711–3717. doi: 10.1128/jb.172.7.3711-3717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Steen M. T., Chung Y. J., Hansen J. N. Characterization of the nisin gene as part of a polycistronic operon in the chromosome of Lactococcus lactis ATCC 11454. Appl Environ Microbiol. 1991 Apr;57(4):1181–1188. doi: 10.1128/aem.57.4.1181-1188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Dahlberg A. E., Atkins J. F., Gesteland R. F. Reading frame switch caused by base-pair formation between the 3' end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J. 1988 May;7(5):1503–1507. doi: 10.1002/j.1460-2075.1988.tb02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


