Abstract
The p38 mitogen-activated protein kinase (MAPK) pathway plays an important role in cell differentiation, but the signaling mechanisms by which it is activated during this process are largely unknown. Cdo is an immunoglobulin superfamily member that functions as a component of multiprotein cell surface complexes to promote myogenesis. In this study, we report that the Cdo intracellular region interacts with JLP, a scaffold protein for the p38α/β MAPK pathway. Cdo, JLP, and p38α/β form complexes in differentiating myoblasts, and Cdo and JLP cooperate to enhance levels of active p38α/β in transfectants. Primary myoblasts from Cdo −/− mice, which display a defective differentiation program, are deficient in p38α/β activity, and the expression of an activated form of MKK6 (an immediate upstream activator of p38) rescues the ability of Cdo −/− cells to differentiate. These results document a novel mechanism of signaling during cell differentiation: the interaction of a MAPK scaffold protein with a cell surface receptor.
Introduction
MAPKs function as the terminal components of three-tiered cascades of kinases comprised of a MAPK kinase kinase (MAP3K), MAPK kinase (MAP2K), and MAPK and are important signal transducers in development, homeostasis, and disease (Chang and Karin, 2001). For example, the p38 subfamily of MAPKs is involved in a wide variety of biological processes, including inflammation, stress responses, and cell differentiation (Zarubin and Han, 2005). The myriad roles of MAPK cascades indicate that the specificity of MAPK activation and function must be regulated. One mechanism by which this occurs is via MAPK scaffold proteins, which are thought to provide (1) specificity between distinct MAPK subfamilies by assembling individual MAPK modules and (2) precise spatial and temporal regulation to MAPK signaling (Morrison and Davis, 2003). How this latter function is accomplished is unclear, but it suggests that scaffold proteins may interact with cell-type specific factors.
Differentiation of cells in the skeletal muscle lineage is coordinated by the family of myogenic bHLH factors (Myf5, MyoD, myogenin, and MRF4; Arnold and Braun, 2000). During differentiation, these proteins work together with additional transcription factors, notably MEF2, to drive muscle-specific gene expression and promote myoblast fusion into myofibers (Molkentin and Olson, 1996; Penn et al., 2004). Tight control of these transcription factors during myogenesis is required, and their activities are regulated by signal transduction pathways. Much evidence indicates that the p38α/β MAPK pathway plays an important role in myogenesis (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000; de Angelis et al., 2005). p38α/β activity increases and persists in differentiating myoblasts, and differentiation is blocked by the p38α/β inhibitor SB203580. p38α/β phosphorylates several proteins involved in muscle-specific gene expression, including MEF2 isoforms, the myogenic bHLH heterodimeric partner E47, the SWI–SNF complex subunit BAF60, and the RNA decay–promoting factor KH-type splicing regulatory protein (Wu et al., 2000; Simone et al., 2004; Briata et al., 2005; Lluis et al., 2005). MAPKs are generally activated in response to extracellular stimuli, and many such cues that activate p38 during inflammatory and other responses have been identified (Dong et al., 2002). However, despite the attention p38α/β has received as a modulator of myogenesis, the signaling mechanisms by which it is activated during this process are largely unknown.
Cdo is a cell surface Ig superfamily member with a long intracellular region (Krauss et al., 2005). Cdo promotes myogenesis in vivo and in vitro; mice lacking Cdo display delayed skeletal muscle development, and primary myoblasts (satellite cells) obtained from such animals differentiate defectively in vitro, expressing reduced levels of muscle-specific proteins and producing myotubes very inefficiently (Cole et al., 2004). Cdo functions in myoblasts as a component of multiprotein complexes that also include the closely related factor Boc, the Ig superfamily receptor neogenin and its ligand netrin-3, and the adhesion molecules N- and M-cadherin (Krauss et al., 2005). Experiments with myoblast cell lines and reporter assays in fibroblasts indicate that one way Cdo promotes myogenesis is to signal to posttranslationally activate myogenic bHLH factors in a fashion that requires its intracellular region (Cole et al., 2004; Krauss et al., 2005). We report that the Cdo intracellular region binds JLP, a scaffold protein for the p38α/β MAPK pathway (Lee et al., 2002; Kelkar et al., 2005). Cdo and JLP cooperate to activate p38α/β in transfectants, and endogenous Cdo, JLP, and p38α/β form complexes during myoblast differentiation. Cdo −/− satellite cells are deficient in their ability to activate p38α/β, and their defective differentiation phenotype is rescued by the expression of an activated form of a p38 MAP2K, MKK6. Thus, one way p38α/β is activated during myogenesis is through the interaction of a pathway-specific scaffolding module with a Cdo-containing cell surface complex.
Results and discussion
Cdo interacts with JLP
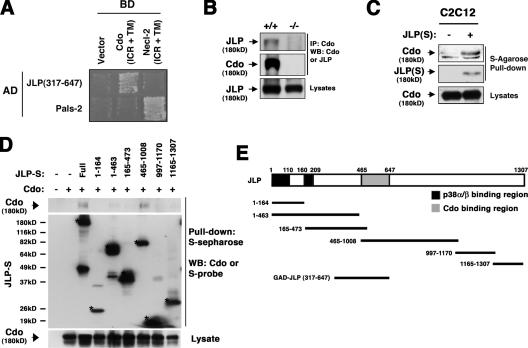
To identify proteins that interact with the Cdo intracellular region, a yeast two-hybrid screen was performed with a construct containing the Cdo transmembrane plus intracellular region as bait (Fig. 1 A). Several positive clones corresponded to a portion of JLP. The transmembrane plus intracellular region of a different Ig protein, Necl-2, was used as a control bait, and JLP did not interact with Necl-2. Conversely, a Necl-2–binding protein, Pals2 (Shingai et al., 2003), bound the Necl-2 bait but not the Cdo bait. JLP, JIP4, and SPAG9 are alternatively spliced products of a single gene (Kelkar et al., 2005), and RT-PCR analysis suggested that JLP is the major product in C2C12 myoblasts (unpublished data). To assess whether Cdo and JLP interact in mammalian cells, lysates from Cdo +/+ and Cdo −/− satellite cells were immunoprecipitated with antibodies to Cdo and blotted for the presence of JLP (Fig. 1 B). JLP was detected in immunoprecipitates from Cdo +/+ but not Cdo −/− lysates, indicating that Cdo was required to bring down JLP and that the antibody was specific. In a reciprocal experiment, C2C12 myoblasts were transiently transfected with an expression vector encoding S epitope-tagged JLP or a control vector, and lysates were precipitated with anti-S agarose (Fig. 1 C). Full-length endogenous Cdo was coprecipitated from the JLP transfectants but not the control transfectants. We concluded that Cdo and JLP interact in myoblasts.
Figure 1.
Cdo interacts with JLP. (A) Yeast transformed with the indicated vectors for Gal4 DNA-binding domain (BD) fused to the transmembrane (TM) plus intracellular regions (ICR) of Cdo or Necl-2 and Gal4 activation domain (AD) fused to a portion of JLP or Pals2 were plated on two-hybrid interaction-dependent selective medium. (B) Lysates from satellite cells of the indicated Cdo genotype were immunoprecipitated (IP) and Western blotted (WB) as indicated. (C and D) Lysates of C2C12 (C) or COS (D) cells transfected with S-tagged JLP derivative or control (−) expression vectors were pulled down with anti-S beads and blotted as indicated for Cdo or S epitope. Asterisks in D indicate S-JLP bands of the predicted size. Numbers above lanes correspond to the JLP amino acids in individual fragments, which are shown schematically in E. (E) S-JLP derivatives from D and the yeast two-hybrid clone (GAD-JLP) are shown to identify the Cdo-binding region of JLP. The p38α/β-binding region of JLP is also shown.
Coimmunoprecipitation experiments in COS cells were then used to identify regions of JLP and Cdo involved in binding. A series of S-tagged fragments of JLP were coexpressed with Cdo and lysates pulled down with anti-S agarose (Fig. 1 D). Only a fragment encoding amino acids 465–1,008 coprecipitated Cdo; because a positive yeast two-hybrid clone contained JLP amino acids 317–647 (Fig. 1 A), the major Cdo-binding region of JLP resides between amino acids 465–647. p38α/β binds to two sites within JLP (amino acids 1–110 and 160–209; Lee et al., 2002), neither of which overlaps the Cdo-binding region (Fig. 1 E), suggesting that Cdo, JLP, and p38 could form a ternary complex. An analogous experiment was performed with Cdo deletion mutants that lack portions of the intracellular region. Loss of JLP binding was seen in each case (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200608031/DC1). It is possible that multiple regions of the Cdo cytoplasmic domain are required to provide structural integrity sufficient for JLP binding or that the Cdo deletion mutants may not be targeted to an appropriate subcellular compartment for interaction.
Cdo-p38 signaling
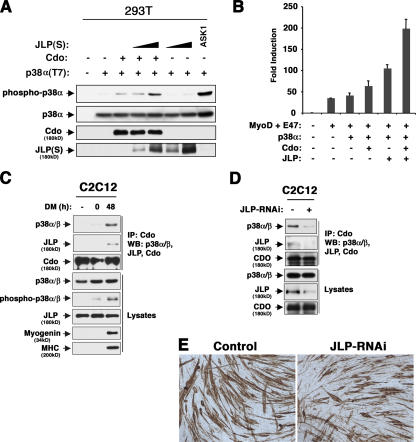
To begin to determine whether Cdo–JLP interaction has a positive effect on p38α/β activity, transfections in heterologous systems were used. 293T cells were transfected with a vector encoding T7-tagged p38α and various combinations of other expression vectors, and lysates were blotted with antibodies to the dually phosphorylated (active) form of p38α/β (pp38α/β; Fig. 2 A). The expression of p38α alone resulted in very low levels of pp38α, but the coexpression of ASK1 (a p38 MAP3K) produced abundant pp38α. The expression of Cdo increased the levels of pp38α above that seen with p38α alone, and this was further increased in a dose-dependent manner by the coexpression of JLP. The expression of JLP without Cdo was less effective. Cdo–JLP interaction was also tested with a MyoD-dependent reporter gene assay in fibroblasts, in which an activated form of the p38 MAP2K, MKK6 (MKK6EE), enhances MyoD activity (Wu et al., 2000). Although the cotransfection of Cdo or JLP separately each increased MyoD-dependent reporter activity above what p38α alone produced, cotransfection of the two together produced ∼80% above what would be expected from a purely additive response (Fig. 2 B). Although the effects of Cdo and JLP coexpression on p38α activity in these heterologous systems are relatively modest, they are clearly stimulatory.
Figure 2.
Cdo, JLP, and p38α/β interact during myogenesis. (A) 293T cells were transfected with the indicated expression vectors and lysates and were Western blotted as indicated. (B) 10T1/2 cells were transfected with the indicated expression vectors plus 4Rtk-luc reporter and, as a control, CMV-lacZ reporter. Luciferase activity was normalized to β-galactosidase activity, and data are reported as the fold induction over the activity of transfectants receiving expression vectors lacking inserts (−). Values are means of duplicate determinations ± SD (error bars). The experi ment was performed three times with similar results. (C) C2C12 cells were cultured in GM (−), at the time of shift to DM (0), or 48 h later, and lysates were immunoprecipitated and Western blotted as indicated. (D) C2C12 cells were transfected with control (−) or JLP RNAi expression vectors, transfectants were sorted, and lysates were immunoprecipitated and Western blotted as indicated. (E) Cells from D were cultured in DM, fixed, and stained for the expression of MHC to reveal myotube formation.
We next asked whether endogenous Cdo, JLP, and p38α/β could be found in complexes during myogenesis. C2C12 cells were harvested while proliferating in growth medium (GM), at the time of transfer to differentiation medium (DM), and 48 h after transfer, when they were differentiating. Lysates were immunoprecipitated with Cdo antibodies and blotted for JLP, p38α/β, and Cdo; whole lysates were also probed for the expression of total p38, JLP, pp38α/β, and the differentiation markers myogenin and myosin heavy chain (MHC; Fig. 2 C). JLP and p38α/β coprecipitated with Cdo only when the cells were actively differentiating despite the fact that total levels of all three proteins were unchanged in the various conditions examined. Furthermore, the formation of this complex correlated with pp38α/β production. It is likely that p38α/β coprecipitates with Cdo via binding to JLP, which interacts with Cdo, as p38α and Cdo did not interact in the yeast two-hybrid system (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200608031/DC1). To address this notion more directly, an RNAi approach was used. The pSilencer vector containing a sequence corresponding to mouse Jlp or an irrelevant sequence (as a control) was cotransfected into C2C12 cells with a GFP expression vector, and the cultures were sorted for the presence of GFP. Sorted cultures were replated and subsequently transferred into DM for 48 h, at which point whole lysates and lysates immunoprecipitated with Cdo antibodies were blotted for JLP, p38α/β, and Cdo (Fig. 2 D). RNAi-mediated knockdown of JLP led to a substantial decrease in the amount of p38α/β that coprecipitated with Cdo even though total levels of p38 were unaffected. Collectively, these results argue in favor of a model in which Cdo binds to JLP and JLP binds to p38α/β. Importantly, C2C12 cells that expressed RNAi to Jlp differentiated less efficiently than control transfectants as measured by myotube formation (fusion indices: control cells = 61.7 ± 4.2% and Jlp RNAi-expressing cells = 31.3 ± 5.9%; Fig. 2 E).
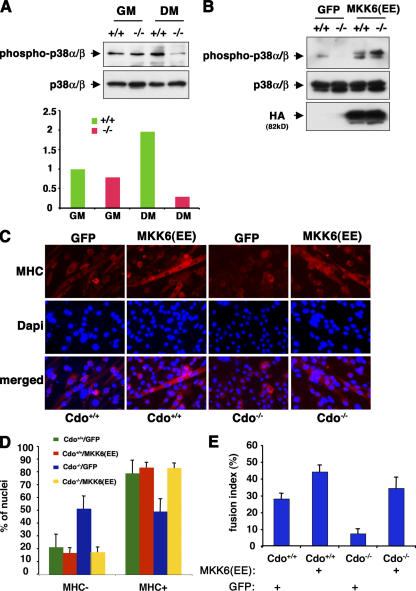
To assess the effect of Cdo loss on p38α/β activity during myogenesis, pp38α/β levels were examined in Cdo +/+ and Cdo −/− satellite cells in GM and DM (Fig. 3 A). Unlike C2C12 cells, satellite cells must be cultured in basic FGF to remain in a proliferative nondifferentiated state, and this is associated with production of a pool of pp38α/β that is thought to target substrates distinct from those involved in differentiation (Jones et al., 2005). Both Cdo +/+ and Cdo −/− satellite cells produced roughly similar amounts of pp38α/β when cultured in GM/basic FGF. As expected, Cdo +/+ cells increased their relative pp38α/β levels when cultured in DM; in contrast, Cdo −/− cells failed to increase or even maintain pp38α/β levels in DM, resulting in an approximately sixfold lower level of pp38α/β than Cdo +/+ cells.
Figure 3.
MKK6EE rescues the differentiation-defective phenotype of Cdo−/− satellite cells. (A, top) Lysates from satellite cells of the indicated Cdo genotype cultured in GM or DM were Western blotted as indicated. (bottom) The image was quantified by densitometry with the pp38α/β signal normalized to the p38α/β signal. Units are arbitrary with Cdo +/+ cells in GM set to 1. The experiment was repeated three times with similar results. (B) Lysates from satellite cells of the indicated Cdo genotype infected with GFP- or HA-tagged MKK6EE-expressing adenoviruses were placed into differentiation-inducing conditions and Western blotted as indicated. (C) Cells as in B were stained for MHC expression (red) and with DAPI to stain nuclei (blue). (D) Quantification of the percentage of nuclei in MHC+ and MHC− cells in cultures of the indicated types. (E) Quantification of the percentage of nuclei in multinucleated cells (fusion index) in cultures of the indicated types. The experiment was repeated three times with similar results. (D and E) Values are means of triplicate determinations ± SD (error bars).
If this lack of differentiation-associated pp38α/β was causally involved in the defective myogenic phenotype of Cdo −/− cells, it would be predicted that the restoration of p38α/β activity at a point downstream of Cdo would rescue the differentiation of these cells. To test this hypothesis, Cdo +/+ and Cdo −/− cells were infected with recombinant adenoviruses encoding either MKK6EE or, as a control, GFP. As seen with uninfected cultures, Cdo +/+ cells infected with the GFP virus had higher levels of pp38α/β than did GFP vector–infected Cdo −/− cells; however, infection with the MKK6EE virus drove production of abundant pp38α/β in both cell types without altering total p38α/β levels (Fig. 3 B). These cultures were then scored for the expression of MHC and production of multinucleated myotubes when cultured under differentiation conditions for 72 h (Fig. 3, C–E). The percentages of GFP vector–infected Cdo +/+ cells that were MHC+ versus MHC− was ∼80/20, whereas the percentages of similarly infected Cdo −/− cells were ∼50/50. The expression of MKK6EE in Cdo −/− cells restored the 80/20 percent ratio of MHC+ versus MHC− cells seen in Cdo +/+ cells but had little effect on Cdo +/+ cells (Fig. 3 D). Likewise, GFP vector–infected Cdo +/+ cells formed elongated myotubes and had a fusion index more than fourfold higher than Cdo −/− cells, which failed to elongate (Fig. 3, C and E). Infection of Cdo −/− cells with the MKK6EE virus led to the production of elongated myotubes by these cells and raised their fusion index to a level similar to that seen with Cdo +/+ cells, which were much more modestly affected by the expression of MKK6EE (Fig. 3, C and E).
Similar results were obtained in C2C12 myoblasts. Differentiation of these cells was inhibited by the expression of RNAi to Cdo, but production of MHC and multinucleated cells was restored by the coexpression of MKK6EE (Fig. S3, available at http:///www.jcb.org/cgi/content/full/jcb.200608031/DC1). It is concluded that the expression of MKK6EE specifically rescues the defects in myogenic differentiation caused by Cdo deficiency, presumably via restoration of p38α/β activity.
Activation of p38α/β during myogenesis
p38α/β MAPK is established as a promyogenic kinase, but the mechanisms by which it is activated during differentiation are not well understood. Certain soluble signaling factors, including ATP and amphoterin, stimulate p38α/β activity and enhance myogenesis when added exogenously to cultured myoblasts; likewise, the expression of a dominant-negative amphoterin receptor blocks production of pp38α/β and differentiation (Ryten et al., 2002; Sorci et al., 2004). Additionally, MyoD activity stimulates a feed-forward pathway that involves activation of p38 via induction of target genes (Penn et al., 2004). However, in general, the signaling mechanisms underlying p38α/β activation by these factors are not clear nor are they confirmed by genetic loss of function data.
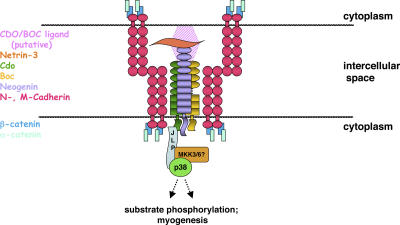
The results described here reveal a novel mechanism of p38α/β activation during myogenesis: the interaction of JLP, a p38α/β MAPK scaffold protein, with Cdo, which is a component of multiprotein cell surface complexes comprised of promyogenic signaling receptors and adhesion molecules (Fig. 4). The binding of a MAPK scaffold protein to the intracellular region of a transmembrane receptor protein is unusual; furthermore, this mechanism is distinct from other known receptor-mediated signaling mechanisms, such as intrinsic enzyme activity (e.g., receptor tyrosine kinases) or direct coupling to nonreceptor tyrosine kinases (e.g., cytokine receptors). JIP3, which is structurally related to JLP, binds the cytoplasmic tail of Toll-like receptor 4 (Matsuguchi et al., 2003), suggesting that direct interaction with transmembrane receptors may be a feature of this class of scaffold protein. It is anticipated that in its role as a scaffold (Lee et al., 2002; Kelkar et al., 2005), JLP brings additional components of the pathway, such as MAP3Ks and MAP2Ks, to these complexes. Furthermore, the interaction of a pathway-specific scaffolding module with Cdo- containing cell surface complexes may allow the coordination of additional signals required for p38α/β activity via the actions of other membrane components of such complexes (e.g., regulation of small GTPases by cadherins; Charrasse et al., 2002). Cdo is also expressed in and promotes the differentiation of neuronal precursors (Zhang et al., 2006b), and similar signaling mechanisms may be involved in myogenesis and neurogenesis. Assembly at sites of cell–cell contact of higher order structures comprised of multiprotein cell surface complexes and intracellular signaling modules is an appealing mechanism for coordinating changes in gene expression and cell morphology during cell differentiation in general.
Figure 4.
Model of Cdo–JLP interaction during myogenic differentiation. A complex of Cdo and its partner proteins positively regulates myogenic differentiation (Krauss et al., 2005). One way this occurs is via the interaction of JLP with the Cdo cytoplasmic tail. JLP is, in turn, bound to p38α/β. This interaction facilitates p38 activation during differentiation and is important for Cdo's effects in myogenesis.
Materials and methods
Yeast two-hybrid screen
A cDNA encoding the transmembrane and intracellular regions of mouse Cdo (amino acids 948–1,250) was fused in frame to the Gal4 DNA-binding domain in the pGBDU-C1 vector. The yeast strain PJ69-4A was sequentially transformed with this vector and a library containing mouse embryo cDNAs fused to the Gal4 activation domain via lithium acetate. Approximately 4.2 × 105 transformants were obtained and screened as described previously (James et al., 1996). Four identical clones encoding amino acids 317–647 of JLP were isolated.
Cell culture
C2C12, 10T1/2, 293T, COS-7, and satellite cells isolated from Cdo +/+ and Cdo −/− mice were cultured as described previously (Cole et al., 2004; Kang et al., 2004). To induce differentiation, C2C12 and satellite cells were transferred into medium containing 2% horse serum or 5% FBS, respectively. Quantification of myotube formation was performed as described previously (Kang et al., 2004).
For RNAi studies shown in Fig. 2, the Jlp sequence 5′-AGATGCGTCTATGAAGCTG-3′ was inserted into the pSilencer 2.0-U6 vector (Ambion). pSilencer 2.0-U6 expressing an irrelevant sequence (Ambion) was used as a control. These vectors were transfected along with a GFP expression vector. Cells were sorted for the presence of GFP and analyzed by coimmunoprecipitation and Western blotting as described in the next paragraph and for the ability to form myotubes. RNAi-mediated knockdown of Cdo was as described previously (Zhang et al., 2006a), and transient assay of its effects on C2C12 differentiation was performed as described previously (Kang et al., 2004). Replication-deficient adenoviruses encoding GFP or HA-tagged MKK6EE were provided by M. Meseck (Mount Sinai School of Medicine, New York, NY) and L. Puri (Burnham Institute, La Jolla, CA; Simone et al., 2004), respectively, and amplified in 293T cells. Satellite cell cultures were infected at an MOI of 50 and assessed for the expression of MKK6EE, pp38α/β, and MHC and for myotube formation.
Protein and reporter assays
Western blot analyses were performed as described previously by Kang et al. (2004). For immunoprecipitations, cells were lysed in extraction buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, and 0.5% Triton X-100 supplemented with 1 tablet/40 ml of Complete protease inhibitor cocktail [Roche]). 2 mg of whole cell extract from each sample was precleared with protein G–Sepharose (GE Healthcare) conjugated with 2 μg of normal rabbit IgG (Santa Cruz Biotechnology, Inc.) for 1 h at 4°C followed by immunoprecipitation with 2 μg anti-Cdo antibody for 2 h at 4°C. Immunocomplexes were washed three times with and suspended in extraction buffer, and samples were analyzed by Western blotting. For pull-down experiments, 4 × 105 cells were seeded onto 100-mm plates 1 d before transfection with plasmids encoding a series of S-tagged JLP proteins (Lee et al., 2002). 2 d after transfection, cells were harvested and lysed in extraction buffer. Whole cell extracts were incubated with 20 μl of 50% slurry S-protein agarose beads (Novagen) for 3.5 h at 4°C. Beads were washed three times with and suspended in extraction buffer, and samples were analyzed by Western blotting.
Antibodies used in this study are as follows: anti-Cdo (Zymed Laboratories), anti-JLP (Abcam), anti-p38α/β (Sigma-Aldrich), anti-pp38α/β (Cell Signaling Technology), anti-T7 (Novagen), anti-S probe (Santa Cruz Biotechnology, Inc.), anti-MHC (MF-20; Developmental Studies Hybridoma Bank), and antimyogenin (Santa Cruz Biotechnology, Inc.).
For reporter assays, 3 × 105 10T1/2 cells were seeded onto individual wells of a six-well plate 1 d before transfection with FuGene6 (Roche). For each well, 200 ng 4RTK-luc and, as an internal control, 100 ng CMV-lacZ plasmids were transfected along with expression vectors for MyoD (50 ng), E47 (10 ng), p38α (100 ng), Cdo (200 ng), and JLP (400 ng) as indicated in Fig. 2 B. Cells were incubated for 48 h after transfection and harvested to determine luciferase and β-galactosidase activity as described previously (Cole et al., 2004; Zhang et al., 2006a).
Microscopy
Cultures were processed as described previously (Kang et al., 2004) and examined on a phase-contrast microscope (Eclipse TS100; Nikon) with plan Fluor 10× NA 0.3 and 20× NA 0.45 objectives (Nikon) at room temperature. Images were captured with a camera (model 2.2.1 Spot RT Color; Diagnostic Instruments) using Spot software (version 3.5.9; Diagnostic Instruments) and Photoshop 7.0 (Adobe).
Online supplemental material
Fig. S1 shows that Cdo deletion mutants do not bind to JLP, and Fig. S2 shows that p38α does not bind to Cdo. Fig. S3 shows that MKK6EE rescues the block to C2C12 cell differentiation imposed by RNAi to Cdo. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200608031/DC1.
Supplementary Material
Acknowledgments
We thank L. Puri, K. Irie, Y. Takai, J. Ninomiya-Tsuji, and A. Yoshimura for reagents and advice and J. Hirsch, M. Frasch, and M. Mlodzik for critical reading of the manuscript.
This work was supported by grants from National Institutes of Health (AR46207) and the T.J. Martell Foundation to R.S. Krauss.
G. Takaesu and J.-S. Kang contributed equally to this paper.
G. Takaesu's current address is Division of Molecular and Cellular Immunology, Medical Institute of Bioregulation, Kyushu University, Fukuoka 812-8582, Japan.
Abbreviations used in this paper: DM, differentiation medium; GM, growth medium; MHC, myosin heavy chain.
References
- Arnold, H.-H., and T. Braun. 2000. Genetics of muscle determination and development. Curr. Top. Dev. Biol. 48:129–164. [DOI] [PubMed] [Google Scholar]
- Briata, P., S.V. Forcales, M. Ponassi, G. Corte, C.Y. Chen, M. Karin, P.L. Puri, and R. Gherzi. 2005. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell. 20:891–903. [DOI] [PubMed] [Google Scholar]
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature. 410:37–40. [DOI] [PubMed] [Google Scholar]
- Charrasse, S., M. Meriane, F. Comunale, A. Blangy, and C. Gauthier-Rouviere. 2002. N-cadherin-dependent cell-cell contact regulates Rho GTPases and b-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, F., W. Zhang, A. Geyra, J.-S. Kang, and R.S. Krauss. 2004. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell. 7:843–854. [DOI] [PubMed] [Google Scholar]
- Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341–4346. [DOI] [PubMed] [Google Scholar]
- de Angelis, L., J. Zhao, J.J. Andreucci, E.N. Olson, G. Cossu, and J.C. McDermott. 2005. Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev. Biol. 283:171–179. [DOI] [PubMed] [Google Scholar]
- Dong, C., R.J. Davis, and R.A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55–72. [DOI] [PubMed] [Google Scholar]
- James, P., J. Halladay, and E.A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, N.C., K.J. Tyner, L. Nibarger, H.M. Stanley, D.D. Cornelison, Y.V. Fedorov, and B.B. Olwin. 2005. The p38α/β MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 169:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.-S., M.-J. Yi, W. Zhang, J.L. Feinleib, F. Cole, and R.S. Krauss. 2004. Netrins and neogenin promote myotube formation. J. Cell Biol. 167:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar, N., C.L. Standen, and R.J. Davis. 2005. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 25:2733–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss, R.S., F. Cole, U. Gaio, G. Takaesu, W. Zhang, and J.S. Kang. 2005. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J. Cell Sci. 118:2355–2362. [DOI] [PubMed] [Google Scholar]
- Lee, C.M., D. Onesime, C.D. Reddy, N. Dhanasekaran, and E.P. Reddy. 2002. JLP: a scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors. Proc. Natl. Acad. Sci. USA. 99:14189–14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis, F., E. Ballestar, M. Suelves, M. Esteller, and P. Munoz-Canoves. 2005. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi, T., A. Masuda, K. Sugimoto, Y. Nagai, and Y. Yoshikai. 2003. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 22:4455–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin, J.D., and E.N. Olson. 1996. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc. Natl. Acad. Sci. USA. 93:9366–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, D.K., and R.J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91–118. [DOI] [PubMed] [Google Scholar]
- Penn, B.H., D.A. Bergstrom, F.J. Dilworth, E. Bengal, and S.J. Tapscott. 2004. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18:2348–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryten, M., P.M. Dunn, J.T. Neary, and G. Burnstock. 2002. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J. Cell Biol. 158:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai, T., W. Ikeda, S. Kakunaga, K. Morimoto, K. Takekuni, S. Itoh, K. Satoh, M. Takeuchi, T. Imai, M. Monden, and Y. Takai. 2003. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 278:35421–35427. [DOI] [PubMed] [Google Scholar]
- Simone, C., S.V. Forcales, D.A. Hill, A.N. Imbalzano, L. Latella, and P.L. Puri. 2004. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36:738–743. [DOI] [PubMed] [Google Scholar]
- Sorci, G., F. Riuzzi, C. Arcuri, I. Giambanco, and R. Donato. 2004. Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol. Cell. Biol. 24:4880–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., P.J. Woodring, K.S. Bhakta, K. Tamura, F. Wen, J.R. Feramisco, M. Karin, J.Y. Wang, and P.L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20:3951–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. [DOI] [PubMed] [Google Scholar]
- Zetser, A., E. Gredinger, and E. Bengal. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274:5193–5200. [DOI] [PubMed] [Google Scholar]
- Zhang, W., J.-S. Kang, F. Cole, M.J. Yi, and R.S. Krauss. 2006. a. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell. 10:657–665. [DOI] [PubMed] [Google Scholar]
- Zhang, W., M.-J. Yi, F. Cole, R.S. Krauss, and J.-S. Kang. 2006. b. Cortical thinning and hydrocephalus in mice lacking the Ig superfamily member CDO. Mol. Cell. Biol. 26:3764–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.