Abstract
The double-stranded RNA-binding motif (dsRBM) is a widely distributed motif frequently found within proteins with sequence non-specific RNA duplex-binding activity. In addition to the binding of double-stranded RNA, some dsRBMs also participate in complex formation via protein–protein interactions. Interestingly, a lot of proteins containing multiple dsRBMs have only some of their dsRBMs with the expected RNA duplex-binding competency proven, while the functions of the other dsRBMs remain unknown. We show here that the dsRBM1 of RNA helicase A (RHA) can cooperate with a C-terminal domain of proline-rich content to gain novel nucleic acid-binding activities. This integrated nucleic acid-binding module is capable of associating with the consensus sequences of the constitutive transport element (CTE) RNA of type D retrovirus against RNA duplex competitors. Remarkably, binding activity for double-stranded DNA corresponding to the consensus sequences of the cyclic-AMP responsive element also resides within this composite nucleic acid binder. It thus suggests that the dsRBM fold can be used as a platform for the building of a ligand binding module capable of non-RNA macromolecule binding with an accessory sequence, and functional assessment for a newly identified protein containing dsRBM fold should be more cautious.
INTRODUCTION
The double-stranded RNA-binding motif (dsRBM) is composed of ∼70 amino acids with consensus sequences scattered throughout the core (1–5). Studies from several RNA-binding competent dsRBMs showed they were highly specific for binding of double-stranded RNA (dsRNA) with little or no detectable binding ability to single-stranded RNA (ssRNA), single-stranded DNA (ssDNA) or double-stranded DNA (dsDNA) (1,4,6,7). However, RNAs with higher order structure such as VAI RNA of adenovirus (8,9) have been found to interact with the two dsRBMs of dsRNA-activated protein kinase (PKR). All the solved three-dimensional structures of dsRBM take an αβββα topology with conserved hydrophobic residues comprised in a hydrophobic core, while the RNA-contacting residues are exposed on the surface (10–14). Structural studies of complexes formed between several dsRBMs and RNA duplexes have revealed direct and water-mediated interactions between conserved protein residues and phosphodiester backbones as well as 2′-OH groups of RNA duplex with limited numbers of base-specific interactions (12,13). This is consistent with the idea that dsRBM binds dsRNA in a sequence non-specific way and is capable of discriminating against DNA. Recently, the fifth dsRBM of Drosophila staufen protein, which is incapable of binding dsRNA was shown to interact with Miranda protein in mediating asymmetric protein and mRNA localization in the developing nervous system (15). In addition, some dsRBMs have been shown to play roles in direct or RNA-mediated protein dimerization (16–20). Therefore, dsRBM fold is capable of mediating specific protein–protein interactions, too.
RNA helicase A (RHA) is the human homolog of Drosophila maleless (MLE) protein with a DEAH box ATPase/helicase domain and is essential for normal gastrulation during mammalian embryogenesis (21–23). Two copies of dsRBM in the N-terminal region and a ssDNA-binding RGG domain at the C-terminus are phylogenetically conserved, and have been proposed to have regulatory functions in the unwinding activity of RHA (24). RHA is a shuttle protein of multiple functions involving mRNA processing and transport (25–28). For example, RHA was demonstrated to play important roles in TAR-mediated HIV-1 gene expression (27). RHA was also found to associate with the constitutive transport element (CTE) RNA of type D retrovirus, a cis-acting viral RNA tag that can override the nucleus retention mechanism for unspliced mRNA in the mammalian host to transport the viral genomic RNA to the cytoplasm for packaging (26,28). The CTE RNA is composed of two domains, loop A and loop B, and each domain contains a symmetric internal loop and an A-rich bulge (29). Chemical and enzymatic mapping of CTE RNA have established that both loop A and loop B of CTE RNA adopt a single-stranded conformation. In addition to RHA, Tip-associated protein (TAP) has also been identified to associate with CTE RNA both in vivo and in vitro (26,30). The CTE RNA-binding unit of TAP is formed by a non-canonical RRM domain and a LRR domain (31,32). Since the RRM domain is a very common RBM for sequence-specific binding of the single-stranded region of a variety of RNA secondary structures (4), it raises the question of how can CTE RNA be recognized by two host factors containing distinct RBMs of different RNA-binding specificity.
In addition to the binding of specific viral RNA motifs, RHA associates with several bio-molecules of different nature (33–36). Recently, RHA was found to participate in transcriptional modulation of p16INK4a tumor suppressor gene by binding to the DNA promoter of p16INK4a gene (33). However, the exact domain within RHA responsible for the p16INK4a gene DNA promoter binding by RHA remains uncharacterized. On the other hand, CREB-binding protein (CBP) and TAP were both shown to interact with RHA, too (34–36). Intriguingly, these interactions between RHA and different macromolecules all involve the N-terminal domain of RHA containing two copies of dsRBM. Together, it suggests that the N-terminal domain of RHA may possess diverse ligand-binding properties in addition to the dsRNA-binding property expected for protein containing dsRBMs.
Although previous works on the issue of nucleic acid-binding activities of RHA have implicated dsRBM involvement in the process, most conclusions were drawn from the experimental results of deletion RHA constructs containing dsRBMs and other domains (24,27,33). For example, the binding of RHA to TAR RNA of HIV was shown to be mediated by the dsRBM2 of RHA (27), whereas previous deletion studies on RHA indicated RHA fragments containing both dsRBMs were capable of sequence non-specific dsRNA binding (24). In order to understand the molecular basis for the duality of CTE RNA recognition by RHA and TAP, we discovered unexpected specific dsDNA-binding activity embedded in the first 120 amino acid residues of RHA. Interestingly, this activity turned out to be related to the recently identified p16INK4a gene DNA promoter binding activity within the N-terminal region of RHA (33). In this report, we will present the progress that has led to this unexpected finding and demonstrate that the dsRBM1 of RHA can cooperate with an extended proline-rich region in its C-terminus to form an integrated nucleic acid-binding module of novel ligand-binding activities. In addition to CTE RNA-binding activity, this integrated unit is also capable of specific dsDNA recognition. Therefore, a modified nucleic acid recognition property can be generated by the construction of a composite unit containing a dsRBM platform and a proximal region. More importantly, our results imply that the widely distributed dsRBM may have the potential to recognize other non-RNA macromolecules by cooperating with the flanking accessory sequences.
MATERIALS AND METHODS
Plasmids
The gene encoding full-length TAP protein was a kind gift from Dr Elisa Izaurralde (30). The recombinant expression vector for glutathione S-transferase (GST)-TAP61–372 was obtained by subcloning the gene fragment spanning residue 61–372 of TAP into the BamHI/EcoRI sites of pGEX-4T1 vector. The gene encoding the first 257 amino acids in the N-terminal domain of RHA was cloned from a human placenta cDNA library (Clontech) with appropriate primers (RHA 5′ sense and RHA 3′ antisense as shown in Table 1) by PCR using pfu DNA polymerase (Promega). The amplified PCR fragments were then inserted into the BamHI/SmaI sites of pGEX-4T1 expression vector (Pharmacia) to make the recombinant pGEX-RHA1–257 vector, which was then used as the parental template for cloning of the genes of various N-terminal deletion mutants of RHA by PCR with suitable primers listed in Table 1. Please note that all the sense and anti-sense primers contain either a BamHI or a SmaI restriction site, and both sites are in bold.
Table 1. The sequences of primers used for PCR amplification of the genes of RHA deletion mutants.
| RHA 5′ sense | 5′-GCG TGG ATC CATGGGTGACGTTAAAAATTTTCTG-3′ |
| RHA 3′ antisense | 5′-TCGACC CGG GTC ATTCAACCACTCCAAGATGGT-3′ |
| RHA1–72 3′ antisense | 5′-AAT TCC CGG GTC ATA TTC GAA CCA AAT AGT T-3′ |
| RHA1–120 3′ antisense | 5′-AAT TCC CGG GTC ATT TGA GAG CCA GAT GTG G-3′ |
| RHA73–180 3′ antisense | 5′-AAT TCC CGG GTC AAT TTT CCA AGG TCC AGT T-3′ |
| RHA180–257 5′ sense | 5′-AAT TGG ATC CAA TGC TAA AGC TCG TCT AAA C-3′ |
| RHA73–180 5′ sense | 5′-AAT TGG ATC CAA TGA AAT AAA GAG TGA A-3′ |
| RHA130–257 5′ sense | 5′-AAT TGG ATC CTC TGG CTA TGG TGT TCC T-3′ |
For the construction of each deletion mutant, the amplified PCR product was ligated into restriction enzymes treated with pGEX-4T1 vector to generate the recombinant vector for expressing GST-tagged RHA deletion protein in Escherichia coli. In contrast, the pMal-2cx expression vector (New England Biolabs) was used as the vehicle for the construction of expression vectors for maltose-binding protein (MBP)-tagged fusion proteins. The primers used for PCR amplification of the RHA1–105 gene were RHA 5′ sense used above and an anti-sense primer of the following sequences, 5′-AATTAAGCTTTCATAAATCTCCTTCAGCATT-3′, containing a HindIII restriction site (in bold). A blue–white screen procedure was performed to identify TB1 colonies carrying the insert with the desired gene according to the manufacturer’s instructions. The recombinant pMal fusion vectors were then recovered and retransformed into competent BL21 (DE3) cells for MBP fusion protein expression after sequence verification. All the mutants with point mutation in RHA1–120 were created by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to manufacturer’s instructions. The identities of all cloned and mutated genes were confirmed by DNA sequencing analysis.
Expression and purification of fusion proteins
Escherichia coli BL21 (DE3) cells were used as the host for expression of GST-tagged RHA and TAP fusion proteins. The transformed bacteria were grown with LB media in the presence of 100 µg/ml ampicillin to an A600 value of 0.6 at 37°C. Expression of GST-tagged proteins was then induced by the addition of 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) and the growth of cells was continued for another 3 h. The cells were then collected by centrifugation and suspended in TGN buffer [20 mM Tris–HCl, 10% glycerol, 0.15 M NaCl, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), pH 8.0] for storage at –80°C. The following procedures were then used to obtain RNase-free and DNase-free proteins suitable for further nucleic acid–protein interaction assays. First, the frozen cell pellets containing expressed GST fusion proteins were thawed and sonicated in the presence of 1 mM PMSF. The cell lysates were centrifuged and the clarified lysates were then applied into a pre-packed glutathione–agarose bead column (Pharmacia) equilibrated with PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3). After extensive washing with 25 column volumes of PBS buffer, the GST-tagged proteins were eluted with 1 column volume of elution buffer of 50 mM Tris–HCl and 10 mM reduced glutathione at pH 8.0. The fractions containing eluted fusion proteins (checked by SDS–PAGE and Coomassie brilliant blue staining) were pooled and dialyzed against buffer containing 1 M ammonium sulfate, 10 mM Na2HPO4, 10 ng/ml pepstatin and 1 mM PMSF at pH 8.0 for 4 h at 4°C. The equilibrated GST fusion proteins were then applied onto a butyl or phenyl-hydrophobic interaction column (Pharmacia) and eluted with a gradient of decreasing concentrations of ammonium sulfate from 1 M to 10 mM in the presence of 10 mM phosphate buffer on an Akata FPLC instrument (Pharmacia). The fractions containing eluted proteins between 0 and 250 mM ammonium sulfate were collected and pooled for concentration by centricon 10 (Millipore). Finally, the concentrated proteins were dialyzed against the binding buffer (see below) and stored as separate aliquots at –80°C. The protein purity was examined by SDS–PAGE, while the protein concentration was measured using the Bradford assay (BioRad).
MBP-tagged fusion proteins were expressed in BL21 (DE3) cells in LB media enriched with 0.2% glucose. The cells were first grown to an A600 value of 0.8, and the fusion protein expression was induced with 1 mM IPTG. The growth of cells was stopped 2 h after induction and then harvested as described above. The expressed MBP fusion proteins were purified by passing the clarified cell lysates through maltose-embedded agarose beads (New England Biolabs) and washed with 10 column volumes of column buffer containing 20 mM Tris–HCl, 200 mM NaCl, 1 mM EDTA, 1 mM sodium azide and 1 mM DTT, and eluted with a column buffer containing 10 mM maltose. Further purification of the MBP fusion proteins was also carried out using a hydrophobic interaction column with procedures similar to those described for the purification of GST-tagged proteins. All the protein purification procedures were operated at under 4°C.
Preparation of RNA
Synthetic RNA used in this study was transcribed by T7 RNA polymerase with designed DNA templates and a top-strand T7 promoter using an in vitro transcription method. After purification using denaturing gel electrophoresis in the presence of 8 M urea, the gels of bands containing the RNA of desired sequence were cut out and electro-eluted using a BIOTRAP device (Schleicher and Schuell). The eluted RNA was then ethanol precipitated and recovered by centrifugation. Finally, the concentrations of RNA were determined by UV absorbance at 260 nm.
Analysis of nucleic acid–protein interactions
The 32P-labeling of RNA at the 5′ end was initiated by the treatment of RNA with shrimp phosphatase (USB-Amersham) at 37°C for 30 min. After the inactivation of phosphatase by incubation at 70°C for 10 min, [γ-32P]ATP (Amersham) and T4 polynucleotide kinase (New England Biolabs) were then added to generate the 5′ 32P-labeled RNA. The labeled RNA was purified using a 20% denaturing polyacrylamide gel and recovered by crushing and soaking procedures. After concentrating by ethanol precipitation, the labeled RNA was recovered by centrifugation. The 5′ 32P-labeling of DNA was carried out with similar procedures as those of 5′-end RNA labeling except that no dephosphorylation step using phosphatase treatment was performed. Labeled RNA probes (10 000 c.p.m. per reaction) with various amounts of protein were incubated for 15 min on ice in a final volume of 10 µl of binding buffer [10 mM Tris–HCl (pH 7.9), 50 mM KCl, 1 mM dithiothreitol, 5 mM MgCl2, 100 ng/ml leupstatin, 10 ng/ml pepstatin, 1 mM PMSF, 1mM EDTA, 10% glycerol in the presence of 20 ng/ml poly(rI·rC) and 150 ng/ml BSA]. The RNA–protein complexes were then analyzed on a 6% non-denaturing polyacrylamide gel (29:1 acryl:bisacryl ratio) in 1× Tris-glacial acetic acid–EDTA (TAE) running at a constant voltage of 150 V with 1× TAE in a 4°C cold box. The results of electrophoretic mobility shift assays (EMSAs) were visualized by autoradiography using a BAS-1500 bioimaging analyzer (Fujifilm). The EMSA for the DNA–protein interaction was performed in a similar way as the RNA–protein interaction assay described above. The DNA competition efficiency, expressed as percent DNA bound, was calculated from the measured optical density of the bands corresponding to the bound and free cyclic-AMP responsive element (CRE) dsDNA. The formula used for the calculation is: [bound / (bound + free)] / [boundc / (boundc + freec)] × 100, where c represents the condition with no competitor added.
RESULTS
Both the dsRBM-containing domain of RHA and the RRM-containing domain of TAP associate with consensus CTE RNA probe in the presence of dsRNA competitor
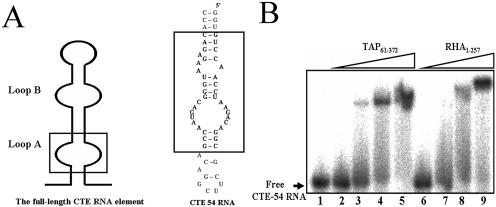
To investigate how two proteins with RBMs of different RNA-binding preferences can target to the same CTE RNA molecule, a truncated CTE RNA domain, CTE-54 RNA (Fig. 1A), corresponding to the loop A of full-length CTE RNA competent for nuclear export (37), was used as the probe for the RNA–protein interaction assay. In addition to the conserved symmetric internal loop, which has been shown to form a conformation of non-helical nature by chemical and enzymatic mapping (28), CTE-54 RNA also carries the all-adenosine asymmetric internal loop and three single- nucleotide bulges that can interrupt the formation of an extended duplex in the lower stem of CTE-54 RNA. Because the CTE RNA is an RNA molecule of 154 nt with multiple structural domains and the exact binding regions in CTE RNA by RHA and TAP are unclear, it is thus possible that TAP and RHA recognize different structural features of CTE RNA. We rationalized that if both TAP and RHA still interact with CTE-54 RNA, it will be less likely that distinct domains in CTE RNA are recognized by TAP and RHA, respectively. A side by side comparison for CTE-54 RNA-binding between the RNA-binding domain of TAP, TAP61–372 and the N-terminal region of RHA, RHA1–257 with dsRNA as competitor by EMSA revealed Kd values in the sub-micromolar range for both CTE-binding proteins (Fig. 1B). Since RHA1–257 contains two copies of well defined dsRBM that should prefer the binding of dsRNA, the observed association between RHA1–257 and CTE-54 RNA in the presence of an excess amount of dsRNA competitors, thus suggested that the domain with a nucleic acid-binding property different from that of a typical dsRBM might be responsible for the observed CTE-54 RNA-binding activity of RHA1–257. Therefore, we decided to turn our focus on further analysis of RHA1–257.
Figure 1.
Direct binding assay of CTE-54 RNA by TAP and RHA. (A) Schematic presentation of full-length CTE RNA showing both loop A and loop B and the predicted secondary structure of CTE-54 RNA. The consensus sequences within loop A and loop B that occur in CTE-54 RNA are boxed. (B) Complex formation between CTE-54 RNA and TAP61–372, and between CTE-54 RNA and RHA1–257 in the presence of RNA duplex competitors [in each binding reaction the final concentration for poly(rI·rC) is 20 ng/µl]. Four different protein concentrations, 0.5 (lanes 2 and 6), 1 (lanes 3 and 7), 2.5 (lanes 4 and 8) and 5 µM (lanes 5 and 9), were used to examine the interaction between 32P-labeled CTE-54 RNA and TAP61–372 or RHA1–257, respectively.
Determination of sequences in the N-terminal region of RHA for efficient CTE-54 RNA binding
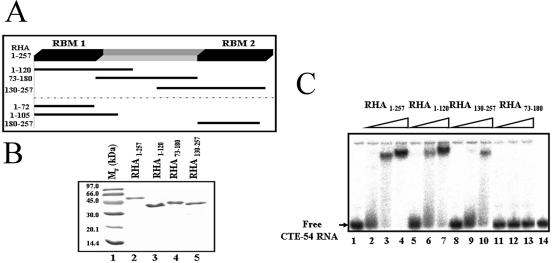
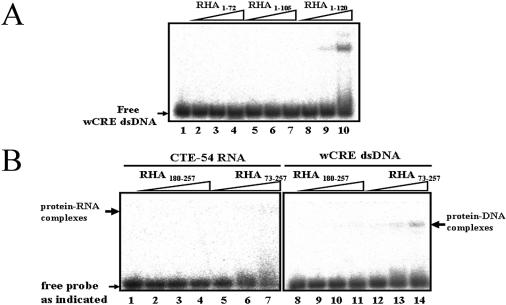
In order to define sequences in the N-terminal region of RHA responsible for CTE-54 RNA recognition, we constructed a series of GST-tagged deletion mutants spanning the first 257 amino acids of RHA (Fig. 2A and B). EMSA of purified GST-tagged RHA deletion mutants revealed that deletion fragments containing either dsRBM1 or dsRBM2 (RHA1–120 and RHA130–257, respectively) were capable of CTE-54 RNA binding although they both showed reduced RNA-binding affinities compared with that of RHA1–257 containing both dsRBMs (Fig. 2C). On the contrary, no detectable binding to CTE-54 RNA was observed for RHA73–180 possessing neither dsRBM1 nor dsRBM2 (lanes 11–13), indicating that both dsRBMs in RHA make a contribution to the binding of CTE-54 RNA by RHA1–257. It also revealed that the isolated dsRBM1-containing RHA1–120 was capable of efficient CTE-54 RNA binding, suggesting that the RNA-binding property of dsRBM1 may be different from that of a typical dsRBM. Since the interaction between RHA and TAR RNA was shown to be mediated by dsRBM2 and not the dsRBM1 of RHA (27), it prompted us to focus on the dsRBM1-containing RHA1–120 to further analyze the role of dsRBM1 in CTE-54 RNA binding by RHA1–120. However, a direct binding affinity comparison between dsRBM1 and RHA1–120 for CTE-54 RNA binding was hindered because the GST-tagged deletion mutant containing only dsRBM1, GST–RHA1–72, was insoluble in aqueous solution. An alternative fusion tag, MBP was thus used to replace GST to generate a soluble dsRBM1-containing protein, MBP–RHA1–72 (see Figs 2A and 3A). Unexpectedly, EMSA data indicated that MBP–RHA1–72 possessed much weaker binding affinity towards CTE-54 RNA compared with that of RHA1–120 in the three different concentrations examined (lanes 2–4 in Fig. 3B).
Figure 2.
Determination of sequences in RHA1–257 needed for efficient CTE-54 RNA binding. (A) Schematic representation of the expressed protein fragments covering residue 1 to residue 257 of RHA. Please note that the three deletion constructs listed below the dashed line were MBP-tagged, while the other deletion constructs above the dashed line were GST-tagged. (B) Purified GST-tagged deletion mutants of RHA separated in a 12% SDS–PAGE and stained with Coomassie brilliant blue. For each well, 3 µg of molecular weight marker (lane 1) or purified deletion protein (lanes 2–5) was loaded. (C) RHA1–120 is capable of efficient CTE-54 RNA binding. 32P-labeled CTE-54 RNA was incubated with increasing amounts of different RHA deletion mutants in the presence of a final concentration of 20 ng/µl poly(rI·rC) competitor and 150 µg of BSA, and analyzed in a 6% non-denaturing polyacrylamide gel. Three different protein concentrations were tested for each mutant with a concentration range of 0.5 (lanes 2, 5, 8 and 11), 2.5 (lanes 3, 6, 9 and 12) and 5 µM (lanes 4, 7, 10 and 13), respectively. Lane 1 contained free CTE-54 RNA only, and lane 14 contained 3 µg of purified GST as control.
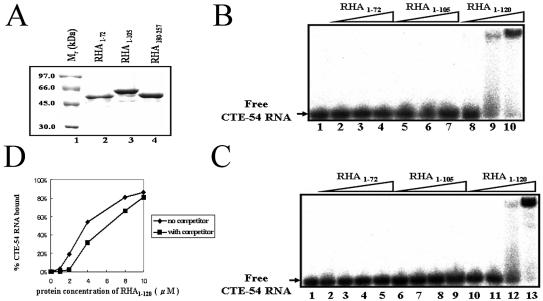
Figure 3.
Both dsRBM1 and the region comprising residues 73–120 are required for efficient CTE-54 RNA binding by RHA1–120. (A) 12% SDS–PAGE for 3 µg of purified MBP fusion proteins stained with Coomassie brilliant blue. Lanes 2–4 contained MBP-tagged fusion protein with their identities shown on top of the gel. (B) CTE-54 RNA binding assay for different MBP fusion protein constructs of three different concentrations, ranging from 0.5 (lanes 2, 5 and 8), 2.5 (lanes 3, 6 and 9) to 5 µM (lanes 4, 7 and 10) in the presence of a final concentration of 20 ng/µl poly(rI·rC) RNA competitor. (C) CTE-54 RNA binding assay for different MBP fusion protein constructs of four different concentrations, 0.5 (lanes 2, 6 and 10), 1.0 (lanes 3, 7 and 11), 2 (lanes 4, 8 and 12) and 10 µM (lanes 5, 9 and 13), in the absence of RNA competitor. (D) Plot of the fraction of CTE-54 RNA bound as a function of protein concentration of RHA1–120 in the absence and presence of RNA competitor. The data were analyzed by performing volume integration of the bands corresponding to free RNA, bound RNA and background sites using a BAS-1500 bioimaging analyzer (Fujifilm). Each experiment was carried out in triplicate and the plotted values are the average value.
To address the possibility that the use of an incomplete dsRBM1 has led to the impairment in CTE-54 RNA-binding ability observed for MBP–RHA1–72, we created a longer dsRBM1-containing fragment, MBP–RHA1–105 (Fig. 3A). Results from the CTE-54 RNA-binding assay as shown in lanes 5–7 of Figure 3B indicated that MBP–RHA1–105 also possessed very weak binding affinity towards CTE-54 RNA, confirming that dsRBM1 alone is not an efficient CTE-54 RNA binder in the presence of dsRNA competitor. Since RHA73–180 could not bind CTE-54 RNA under the same condition, it thus suggested that RHA73–120 was not capable of efficient CTE-54 RNA binding by itself, either. Together, these results indicated extra sequences (residues 73–120) C-terminal to dsRBM1 were required for efficient CTE-54 RNA binding by RHA1–120.
The dsRBM1-containing RHA1–120 is a composite ligand-binding unit with versatile nucleic acid-binding property
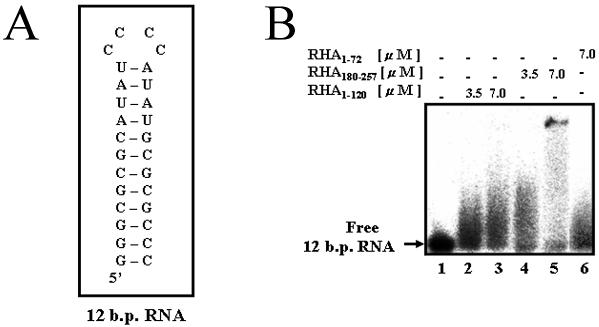
To further investigate the roles of dsRBM1 and residues 73–120 within RHA1–120 for the binding of CTE-54 RNA, we measured the binding affinities of RHA1–120, RHA1–105 and RHA1–72 towards CTE-54 RNA in the absence of the dsRNA competitor using EMSA. As shown in Figure 3C, only the full-length RHA1–120 was capable of efficient CTE-54 RNA binding. Furthermore, no CTE-54 RNA binding was detected in the absence of the dsRNA competitor for RHA73–180 with protein concentrations as high as 10 µM (data not shown). Therefore, RHA1–120 is an integrated CTE-54 RNA-binding unit composed of both dsRBM1 and its carboxyl extended region (residues 73–120), and both components are required for efficient CTE-54 RNA binding. A comparison of CTE-54 RNA binding affinity by RHA1–120 in the presence and absence of the dsRNA competitor revealed that there was no dramatic increment of CTE-54 RNA-binding affinity without the dsRNA competitor (Fig. 3D), implying that dsRNA may not be the preferred RNA ligand for RHA1–120 compared with CTE-54 RNA. Thus, we used an RNA hairpin containing a 12 bp stem, the 12 bp RNA hairpin (Fig. 4A), to evaluate the RNA duplex-binding abilities of dsRBM1, dsRBM2 and RHA1–120. Previously, this same RNA hairpin has been shown to be sufficient for binding by the dsRBM3 of staufen protein in a structural study (13), and studies of the dsRNA-binding property of dsRBM in PKR has also revealed the requirement for a minimum duplex length per protein molecule to be ∼11 bp for stable complex formation (7,38). The results of the interaction assay between the 12 bp RNA hairpin and RHA1–120, RHA1–73 (dsRBM1) and RHA180–257 (dsRBM2) suggested different dsRNA-binding tendencies between RHA1–120 and RHA180–257 (Fig. 4B), and was in agreement with the observed association between RHA1–120 and CTE-54 RNA in the presence of an excess amount of RNA duplex competitor.
Figure 4.
dsRBM2 is a better 12 bp RNA hairpin binder than RHA1–120. (A) Secondary structure of the 12 bp RNA hairpin. (B) EMSA of the 32P-labeled 12 bp RNA hairpin in the presence of different protein fragments without non-specific RNA competitor. The concentration of protein used in each well is indicated at the top of the gel.
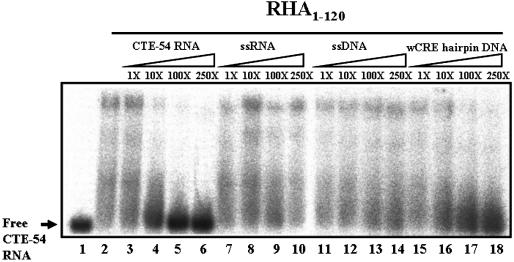
The extended composition requirement for the formation of an efficient RNA-binding unit for RHA1–120 as well as its different binding tendencies toward CTE-54 RNA and 12 bp RNA hairpin implied a distinct nucleic acid-binding property of RHA1–120 from a typical dsRBM. Thus, we asked if the dsRBM1-containing RHA1–120 has the same nucleic acid-binding preference as a typical dsRBM does. To address this question, CTE-54 RNA bound RHA1–120 complex was challenged with an excess amount of unlabeled nucleic acid of different classes, including ssDNA, dsDNA and ssRNA, respectively. In practice, a 21 nt ssDNA corresponding to one strand of SP1 promoter, a 21 bp DNA hairpin containing the consensus sequences of CRE and a 12 nt RNA with the sequences designed to form only a ssRNA conformation were used as the competitors, respectively (see Table 2 for the sequences of competitors used). Remarkably, we found the DNA hairpin could compete with CTE-54 RNA for the binding of RHA1–120 effectively (lanes 15–18 in Fig. 5). In contrast, no competition was observed for the RHA1–120 binding of labeled CTE-54 RNA by the other competitors used except the cold CTE-54 RNA. Therefore, RHA1–120 was not only a composite CTE-54 RNA-binding unit but might also possess a dsDNA-binding property.
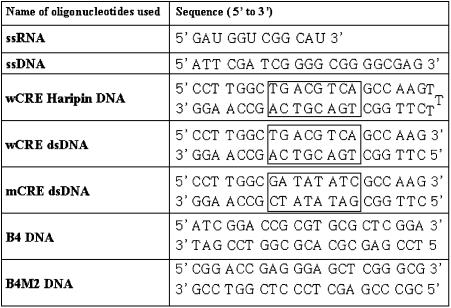
Table 2. Sequences of different nucleic acid probes used in this work.
The boxed regions in wCRE-related sequences represent the consensus CRE sequence. Please note that this consensus sequence has been totally changed in mCRE dsDMA.
Figure 5.
Cold wCRE dsDNA can compete with labeled CTE-54 RNA for the binding of RHA1–120 effectively. RHA1–120 (2.5 µM) was incubated with 0.03 pmol of 32P-labeled CTE-54 RNA in the competition experiments with different folds of cold nucleic acid competitors added as indicated.
The finding that a hairpin with a dsDNA stem was capable of competing with CTE-54 RNA for RHA1–120 binding suggested that dsDNA might be a ligand for RHA1–120. However, the observed competition might be caused by specific structural features formed by the hairpin used in the study. To rule out this possibility, we used a two-stranded complementary DNA duplex corresponding to CRE, wCRE dsDNA as probe (see Table 2 for the sequences of wCRE dsDNA) to examine the dsDNA-binding ability of RHA1–120 directly. In agreement with the results of the competition experiment in Figure 5, the direct binding assay showed that RHA1–120 was capable of binding with wCRE dsDNA as well (lanes 8–10 in Fig. 6A). Further fine mapping of the minimal domain required for wCRE dsDNA binding was carried out using MBP–RHA1–72 and MBP–RHA1–105 constructed above (lanes 2–7). The result shown in Figure 6A indicated that RHA1–120 was also the unit required for efficient wCRE dsDNA binding. To be sure that the association of wCRE dsDNA with RHA1–120 is not the result of an artifact caused by deletion, RHA1–257 was checked for its wCRE dsDNA-binding property, too. We found RHA1–257 also bound wCRE dsDNA (data not shown), reaffirming that the unexpected finding of wCRE dsDNA binding by RHA1–120 is indeed an intrinsic property of the N-terminal regulatory domain of RHA.
Figure 6.
RHA1–120 is sufficient for wCRE dsDNA binding. (A) Fine mapping for the minimal region required for wCRE dsDNA binding. Direct wCRE dsDNA binding by RHA1–72, RHA1–105 and RHA1–120 of 1.25 (lanes 2, 5 and 8), 2.5 (lanes 3, 6 and 9) and 5 µM (lanes 4, 7 and 10) were analyzed by a 6% non-denaturing gel. (B) RHA73–257 possesses higher CTE-54 RNA- and wCRE dsDNA-binding affinity than dsRBM2. In these direct-binding experiments, 32P-labeled CTE-54 RNA (lanes 1–7) was incubated with 0.5, 2.5 and 5 µM of purified dsRBM2 (MBP–RHA180–257) and GST–RHA73–257, respectively (lanes 2–4 and lanes 5–7), while 32P-labeled wCRE dsDNA (lanes 8–14) was incubated with 1.25, 2.5 and 5 µM of purified dsRBM2 (MBP–RHA180–257) and GST–RHA73–257, respectively (lanes 9–11 and lanes 12–14).
After establishing that both dsRBM1 and RHA73–120 are required components for the formation of the efficient CTE-54 RNA and wCRE dsDNA binder, RHA1–120, an interesting question to ask then was if RHA73–120 could help dsRBM2 to form a similar composite ligand binder. Thus, we constructed a deletion mutant, GST–RHA73–257 with RHA73–120 in the N-terminal end to dsRBM2 to see if the addition of RHA73–120 can affect the nucleic acid-binding property of dsRBM2. The CTE-54 RNA- and wCRE dsDNA-binding activity of RHA73–257 were then examined and compared with those of MBP–RHA180–257, containing only dsRBM2. The results shown in Figure 6B did indicate better CTE-54 RNA- and wCRE dsDNA-binding affinities by GST–RHA73–257 than by MBP–RHA180–257, suggesting that RHA73–120 may cooperate with dsRBM2 to form an efficient binder. However, we cannot rule out the possibility that sequences intervening them, namely RHA121–179, may also make a contribution, and attempts at the construction of a chimeric protein with RHA73–120 attached directly to the carboxyl end of dsRBM2 were not successful.
RHA1–120 is capable of recognizing the consensus sequence of CRE dsDNA in vitro
Because the use of wCRE dsDNA as a competitor was a random pick with the DNA oligonucleotides available in our laboratory, it was possible that the observed wCRE dsDNA binding by RHA1–257 and RHA1–120 were just results of the non-specific dsDNA-binding property of this part of RHA. In particular, the N-terminal region of RHA was recently shown to associate with the promoter DNA of INK4a tumor suppressor gene both in vitro and in vivo (33). It was thus interesting to see if RHA1–120 was also capable of binding with the INK4a promoter DNA. We used a pair of complementary DNA oligonucleotides corresponding to the reported promoter dsDNA of INK4a, B4 (33), to examine the direct B4-binding ability of RHA1–120. As shown in Figure 7A, we found RHA1–120 bound B4 DNA with weaker affinity than that of wCRE dsDNA in the three different concentrations tested (compare with Fig. 6A, lanes 8–10). In contrast, a DNA probe with point mutations on B4, B4M2 (Table 2), showed no sign of significant complex formation in the same experimental condition, suggesting that this interaction seems to be specific, although the affinity is not as good as that of wCRE dsDNA. Further Kd calculations using the same method as that in Figure 3D led to an estimated Kd value of 30 µM for B4 DNA and 6 µM for wCRE dsDNA by RHA1–120 (data not shown). Therefore, the reported INK4a promoter dsDNA binding by RHA and our finding of CRE dsDNA binding by RHA1–120 may be related.
Figure 7.
The interaction between wCRE dsDNA and RHA1–120 is specific. (A) Discrimination of B4 dsDNA from mutated B4 dsDNA, B4M2 dsDNA by RHA1–120. In this direct binding assay, 0.03 pmol of 32P-labeled B4 DNA (lanes 1–4) or B4M2 (lanes 5–8) were incubated with 1.25, 2.5 and 5 µM of purified RHA1–120 (lanes 2–4 and lanes 6–8), respectively. (B) Competition activities of different dsDNA competitors and CTE-54 RNA were analyzed by EMSA. In each well, 2 µM of purified RHA1–120 was incubated with 0.03 pmol of 32P-labeled wCRE DNA and challenged by different folds of cold nucleic acids as indicated. (C) Competition efficiency of different nucleic acids for RHA1–120 plotted as a percentage of CRE dsDNA bound against different folds of competing nucleic acids. All EMSAs were done at least three times, and the percentages of dsDNA bound were determined as described in Materials and Methods. (D) GAC and CGT triplets can be found within consensus CRE dsDNA sequence in wCRE dsDNA as well as the central region of B4 DNA, whereas E.coli purF operator dsDNA contains two CGT triplets. The consensus CRE sequences within wCRE dsDNA are in bold and the GAC and CGT triplets are shaded and underlined, respectively.
To further investigate the specificity issue, we used a series of dsDNA oligonucleotides of 21 bp in length (Table 2) to examine the dsDNA-binding specificity of RHA1–120. The specificity of RHA1–120 for different dsDNAs was studied by the abilities of these competitors to compete for binding to RHA1–120 with the labeled wild-type CRE dsDNA (Fig. 7B). Shown in Figure 7C is the competition efficiencies of different dsDNA and CTE-54 RNA competitors, expressed as percent dsDNA bound (see Materials and Methods). It indicates an order of competition efficiency of CRE dsDNA > CTE-54 RNA > B4 dsDNA > purF operator dsDNA > mCRE dsDNA. Indeed, we found mCRE dsDNA lacked the ability of CRE dsDNA competition at all, even at 100-fold excess. Since the consensus CRE sequence, TGACGTCA from wCRE dsDNA (please note the palindrome nature of this sequence), is completely changed in the corresponding position within mCRE dsDNA, it is thus suggested that the consensus CRE dsDNA sequences contained the major recognition site for RHA1–120. Interestingly, sequence analysis of the B4 dsDNA and E.coli purF operator dsDNA (39) showed that both dsDNA competitors contained 3 bp triplets of different composition with sequences identical to those within the consensus sequences of CRE dsDNA. There are a GAC triplet and a CGT triplet in the central region of B4 dsDNA. In contrast, two CGT triplets can be found in E.coli purF operator dsDNA (Fig. 7D). Together, they explain the observed intermediate competition efficiencies for these two competitors and suggest that RHA1–120 recognizes specific sequences in the consensus sequence of CRE dsDNA.
Recognition of CRE dsDNA and CTE-54 RNA by RHA1–120 involve distinct but overlapped residues
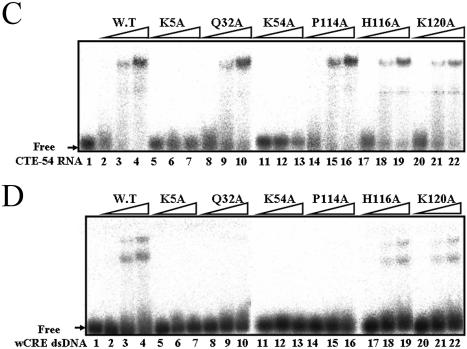
Possession of both CTE-54 RNA- and wCRE DNA-binding capabilities by RHA1–120 raised the question of how could an A-form RNA and a B-form DNA both be recognized by RHA1–120. Competition experiments indicated CTE-54 RNA and CRE dsDNA were not compatible for simultaneous binding to RHA1–120 (Figs 5 and 7B). To further investigate the role of dsRBM1 and the C-terminal region within RHA1–120 for the binding of CTE-54RNA and CRE dsDNA, we created a series of point mutation mutants of GST– RHA1–120 (Fig. 8A) based on published mutagenesis and structure studies on the complexes formed between dsRBMs and model RNA duplexes (12,13). Previous structural studies have revealed that the conserved residues in loop 2 and loop 4 of dsRBM interact with the 2′-OH groups in the minor groove, and the phosphodiester backbones across adjacent major grooves of A-form dsRNA, respectively. In addition, interaction between the single-strand nucleotide in the hairpin loop of a model RNA duplex and the amino acid side chain in the α1 helix of dsRBM was observed from NMR studies (13). As shown in Figure 8B, sequence alignment between several well characterized dsRBMs as well as the dsRBM3 of staufen and dsRBM1 of RHA indicated K5, Q32 and K54 of RHA were in the corresponding positions to the α1 helix, loop 2 and loop 4 of staufen dsRBM3, respectively. Therefore, these three residues were mutated to alanine independently to create K5A, Q32A and K54A mutants of RHA1–120. To probe the roles of the C-terminal region of RHA1–120 in both CTE-54 RNA and CRE dsDNA binding, three mutants including P114A, H116A and K120A were also generated because of the obvious differences in binding affinities towards both nucleic acids between RHA1–105 and RHA1–120. The most informative results were from mutated residues affecting the binding with CRE dsDNA but not affecting the binding affinity for CTE-54 RNA, such as Q32A in the loop 2 of dsRBM1 and P114A in the C-terminal region (compare lanes 8–10 and lanes 14–16 in Fig. 8C with those in D). Therefore, the same residue could play different roles in CTE-54 RNA and CRE dsDNA binding, respectively. It also reaffirmed the requirement of both dsRBM1 and the extended C-terminal region for CRE dsDNA binding. In contrast, K5A and K54A (lanes 5–7 and lanes 11–13, respectively, in Fig. 8C and D) lost both CTE-54 RNA- and CRE dsDNA-binding activities under the same experimental conditions. Since both residues were located on the surface of dsRBM, the loss in both CTE-54 RNA- and CRE dsDNA-binding activities was unlikely to be caused by alteration of domain conformation due to mutagenesis on these two residues. Together, these results suggested that an overlapped but distinct set of residues could participate in CTE-54 RNA- and CRE dsDNA-binding, respectively.
Figure 8.
Two overlapped but distinct sets of residues within RHA1–120 participate in CTE-54 RNA and wCRE dsDNA binding, respectively. (A) Sequence and predicted secondary structure of RHA1–120. The position of the residue mutated to alanine in this study is in red, while the cartoon in the top represents α-helix (spiral), β-sheet (cylinder) and random coil conformation (bolded dashed lines). (B) Sequence alignment for dsRBM1 and dsRBM2 of RHA with other dsRBMs of proven RNA-binding functions. The conserved histidine in loop 2 and conserved lysine in loop 4 of RNA-binding dsRBM are highlighted by gray and black boxes, respectively, while the cartoon in the bottom represents the α-helix (spiral) and β-sheet (cylinder), respectively. (C) The EMSA results for the CTE-54 RNA binding assay of different RHA1–120 mutants. Purified RHA1–120 variants (0.5, 2.5 and 5 µM) as indicated were incubated with 32P-labeled CTE-54 RNA and analyzed using a 6% non-denaturing polyacrylamide gel. (D) The EMSA results for the wCRE dsDNA binding assay of different RHA1–120 mutants. Purified RHA1-120 variants (0.5, 2.5 and 5 µM) as indicated were incubated with 32P-labeled wCRE dsDNA and analyzed using a 6% non-denaturing polyacrylamide gel.
DISCUSSION
A composite nucleic acid-binding unit with dual RNA- and dsDNA-binding activities formed by a dsRBM and a proline-rich domain
Although the dsRBM1 of RHA lacked efficient dsRNA-binding ability that was expected for a typical dsRBM, we found it still played a critical role in CTE-54 RNA binding for a longer RHA fragment containing dsRBM1 as demonstrated by the reduction in CTE-54 RNA-binding ability of RHA1–120 mutants with point mutations within dsRBM1. Distinct CTE-54 RNA-binding affinities between RHA1–72 and RHA1–120 strongly indicated the extra C-terminal residues added into RHA1–72 also possessed key determinant sequences required for the observed CTE-54 RNA-binding property of RHA1–120. However, secondary structure prediction of residues 73–120 by the PHD program (40) suggested this region possessed neither α-helix nor β-sheet properties and was consistent with its high content of proline; prediction by PONDOR also indicated that the residue 73–120 region might adopt a random coil conformation with a high score for intrinsic disorder content (41). Nevertheless, results from the negative CTE-54 RNA-binding observations of both RHA73–180 and the K54A point mutation of RHA1–120 argue that both dsRBM1 and the residue 73–120 region of RHA are required, but not sufficient for, CTE-54 RNA binding by RHA1–120.
We also report here an unexpected wCRE dsDNA-binding ability embedded in the N-terminal region of RHA. This finding is unique because it involves a composite dsDNA-binding unit formed by a dsRBM and a flanking region with a tendency to high flexibility. Furthermore, this RHA1–120 is indeed a dual CTE-54 RNA and CRE dsDNA binder and the binding of RNA and dsDNA are incompatible. Dual RNA- and DNA-binding ability of the same protein has been found in the RRM of murine IPEB protein (42). Complexes formed between nuclear factor 90 and nuclear factor 45 also possess dual RNA- and DNA-binding activities (43–45). Recently, the bacteria histone-like protein HU was shown to bind to dsDNA and dsRNA with similar affinity, and two highly flexible arms of β-ribbon and a α-helical platform were proposed to accommodate the distinct A-form RNA and B-form DNA binding by HU protein (46). More relevant to the current study, a variation of the dsRBM fold with a βββα platform has also been shown to harbor the DNA-binding surface for integrase, although a different surface from the proposed RNA-binding surface in dsRBM was used for dsDNA recognition (47). Besides, flexibility provided by the disorder linker has been proposed to help facilitate docking and locking various targets with different architectures (48). Our results of mutagenesis studies of RHA1–120 suggest that RHA1–120 possesses a composite binding platform with distinct but overlapped recognition surfaces formed by cooperation between dsRBM1 and the predicted highly flexible proline-rich domain for the binding of CRE dsDNA or CTE-54 RNA, respectively. Finally, the generality for the role of RHA73–120 in helping dsRBMs to form a composite ligand binder is not clear based on current data. Further spectroscopic analysis should help in the verification of the boundaries of these binding surfaces as well as the nature and response of this proline-rich region upon the binding of different classes of nucleic acids.
Implications for the function of dsRBM variants in multiple dsRBM-containing proteins and possible functional roles of CRE dsDNA binding by the regulatory domain of RHA
dsRBM was first discovered as a dsRNA-binding motif with conserved residues, and later shown to be capable of discriminating an A-form RNA duplex from a B-form DNA duplex (1–5). In addition, some dsRBMs can self-dimerize or oligomerize upon RNA binding, and protein interaction between dsRBM and other cellular proteins have also been reported recently (15,19,20). Proteins containing dsRBM have been grouped into nine dsRBP families, and most dsRBPs possess multiple dsRBMs (5). Interestingly, the dsRNA-binding affinity of dsRBM varies and some are even RNA-binding incompetent (27,49–53), and the functions of these ‘orphan’ dsRBMs are unclear. Our finding that a dsRBM can cooperate with a C-terminal flanking region to gain CTE-54 RNA- and CRE dsDNA-binding activities, therefore, provides an alternative view in evaluating the function of proteins containing dsRBM. Perhaps, the dsRBM fold is indeed a versatile platform suitable for the construction of various ligand-binding scaffolds in cooperation with different accessory sequences. Since the N-terminal region of RHA is also involved in protein–protein interactions with CBP (34,35) and TAP protein (36), it will be very interesting to see if RHA1–120 also participates in direct protein–protein recognition.
The helicase activity of RHA has been shown to be required for efficient cAMP-mediated transcriptional activation by associating the CBP to RNA polymerase II, and thus may play a role in chromatin remodeling during transcription (34,35). Recently, RHA was also found to participate in transcriptional modulation of p16INK4a tumor suppressor gene by binding to the DNA promoter of p16INK4a gene (33). The finding of residency for wCRE dsDNA-binding activity within the N-terminal regulatory domain of RHA is thus consistent with the common notion that RHA can serve as a DNA helicase for chromatin remodeling during transcription (34,35), although the in vivo function of this CRE dsDNA-binding activity is not clear. Finally, the competition and direct binding assay for RHA1–120 between CRE dsDNA and different dsDNA competitors including B4 dsDNA also imply that the common motif containing CGT and GAC triplets are important for recognition, suggesting that the reported INK4a promoter dsDNA binding by RHA and our finding of CRE dsDNA binding by RHA1–120 may be relevant in terms of the mode of DNA recognition as well as the roles in transcription activation. A possible role for CRE dsDNA binding to the regulatory domain of a DNA helicase is that it may help in targeting the DNA helicase to a specific gene to facilitate the activation of transcription. Alternatively, this CRE dsDNA-binding activity can be irrelevant to the function of helicase activity at all. This is particularly interesting because RHA has also been shown to be involved in the cAMP-dependent transcriptional activation of specific genes by direct protein–protein interaction to CBP (34), and the CRE promoter recognition in the cAMP-responsive pathway is mediated via the bZIP domain of the CRE-binding protein (CREB) (54). Because of the palindrome nature of the consensus sequences, CRE dsDNA harbors two half-sites for dimeric bZip domain recognition. Interestingly, we have noticed that two different DNA–protein complexes were formed in the EMSA analysis between RHA1-120 and wCRE dsDNA (Figs 6A, 7B and 8D). One possible explanation is that they may be caused by the existence of two protein-binding sites in wCRE dsDNA (54). However, further work will be needed to explore the in vivo function and the nature of interactions between the regulatory domain of RHA and the consensus sequences of CRE dsDNA.
Acknowledgments
ACKNOWLEDGEMENTS
We are very grateful to Dr Hung-Wen Chen for the gift of the human placenta cDNA library and Ms Yu-Pine Liu for her contribution in the early stages of this work. We also would like to thank Dr Elisa Izaurralde for the gene of full-length TAP. This work was supported by Grant NSC 91-2311-B-005-038 from the National Science Council of Taiwan.
REFERENCES
- 1.St Johnston D., Brown,N.H., Gall,J.G. and Jantsch,M. (1992) A conserved double-stranded RNA-binding domain. Proc. Natl Acad. Sci. USA, 89, 10979–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S.J., Thomis,D.C. and Samuel,C.E. (1992) Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2a protein kinase. Virology, 188, 47–56. [DOI] [PubMed] [Google Scholar]
- 3.Green S.R. and Mathews,M.B. (1992) Two RNA-binding motifs in the double-stranded RNA activated protein kinase. Genes Dev., 6, 2478–2490. [DOI] [PubMed] [Google Scholar]
- 4.Burd C.G. and Dreyfuss G (1994) Conserved structures and diversity of functions of RNA-binding proteins. Science, 265, 615–621. [DOI] [PubMed] [Google Scholar]
- 5.Fierro-Monti I. and Mathews,M.B. (2000) Protein binding to duplexed RNA: one motif, multiple functions. Trends Biochem. Sci., 25, 241–246. [DOI] [PubMed] [Google Scholar]
- 6.Bass B.L., Hurst,S.R. and Singer,J.D. (1994) Binding properties of newly identified Xenopus proteins containing dsRNA-binding motif. Curr. Biol., 4, 301–314. [DOI] [PubMed] [Google Scholar]
- 7.Bevilacqua P.C. and Cech,T.R. (1996) Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry, 35, 9983–9994. [DOI] [PubMed] [Google Scholar]
- 8.Galabru J., Katze,M.G., Robert,N. and Hovanessian,A.G. (1989) The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur. J. Biochem., 178, 581–589. [DOI] [PubMed] [Google Scholar]
- 9.Mellitis K.H., Kostura,M. and Mathews,M.B. (1990) Interaction of adenovirus BA RNA I with the protein kinase DAI: nonequivalence of binding and function. Cell, 61, 843–852. [DOI] [PubMed] [Google Scholar]
- 10.Bycroft M., Grunert,D., Murzin,A.G. and St Johnston,D. (1995) NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J., 14, 3536–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharrat A., Macias,M.J., Nilges,M. and Pastore,A. (1995) Structure of the dsRNA binding domain of E. coli RNase III. EMBO J., 14, 3572–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryter J.M. and Schultz,S.C. (1998) Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J., 17, 7505–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos A., Grunert,S., Adams,J., Micklem,D.R., Proctor,M.R., Freund,S., Bycroft,M., St Johnston,D. and Varani,G. (2000) RNA recognition by staufen double-stranded RNA-binding domain. EMBO J., 19, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanduri S., Carpick,B.W., Yang,Y., Williams,B.R.G. and Qin,J. (1998) Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J., 17, 5458–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuldt A.J., Adams,J.H.J., Davidson,C.M., Micklem,D.R., Haseloff,J., St Johnston,D. and Brand,A.H. (1998) Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev., 12, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosentino G.P., Venkatesan,S. and Serluce,F.C. (1995) Double-stranded RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc. Natl Acad. Sci. USA, 92, 9445–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel R.C. and Sen,G.C. (1998) Requirement of PKR dimerization mediated by specific hydrophobic residues for its activation by doubled-stranded RNA and its antigrowth effects in yeast. Mol. Cell. Biol., 18, 7009–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano P.R., Zhang,F., Tan,S.L., Garcia-Barrio,M.T., Katze,M.G., Dever,T.E. and Hinnebusch,A.G. (1998) Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol., 18, 7304–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian B. and Mathews,M.B. (2001) Functional characterization of and cooperation between the double-stranded RNA-binding motifs of the protein kinase PKR. J. Biol. Chem., 276, 9936–9944. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F., Romano,P.R., Nagamura-Inoue,T., Tian,B., Dever,T.E., Mathews,M.B., Ozato,K. and Hinnebusch,A.G. (2001) Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem., 276, 24946–24958. [DOI] [PubMed] [Google Scholar]
- 21.Lee C.G. and Hurwita,J. (1992) A new RNA helicase A isolated from HeLa cells that catalytically translocates in the 3′ to 5′ direction. J. Biol. Chem., 267, 4398–4407. [PubMed] [Google Scholar]
- 22.Lee C.G. and Hurwita,J. (1993) Human RNA helicase A is homologous to the maleless protein of Drosophila. J. Biol. Chem., 268, 16822–16830. [PubMed] [Google Scholar]
- 23.Lee C.G., Costa Soares,V., Newberger,C., Manova,K., Lacy,E. and Hurwita,J. (1998) RNA helicase A is essential for normal gastrulation. Proc. Natl Acad. Sci. USA, 95, 13709–13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S. and Grosse,F. (1997) Domain structure of human nuclear DNA helicase II (RNA helicase A). J. Biol. Chem., 272, 11487–11494. [DOI] [PubMed] [Google Scholar]
- 25.Fujii R., Okamoto,M., Aratani,S., Oishi,T., Ohshima,T., Taira,K., Baba,M., Fukmizu,A. and Nakajima,T. (2001) A role of RNA helicase A in cis-acting transactivation response element-mediated transcriptional regulation of human immunodeficiency virus type 1. J. Biol. Chem., 276, 5445–5451. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Hermann,C. and Grosse,F. (1999) Pre-mRNA and mRNA binding of human nuclear DNA helicase II (RNA helicase A). J. Cell Sci., 112, 1055–1064. [DOI] [PubMed] [Google Scholar]
- 27.Tang H., Gaietta,G.M., Fischer,W.H., Ellisman,M.H. and Wong-Staal,F. (1997) A cellular cofactor for the constitutive transport element of type D retrovirus. Science, 276, 1412–1415. [DOI] [PubMed] [Google Scholar]
- 28.Pasquinelli A., Ernst,R.K., Lund,E., Grimm,C., Zapp,M.L., Rekosh,D., Hammarkskjöld,M.L. and Dahlberg,J.E. (1997) The constitutive transport element (CTE) of Mason–Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J., 16, 7500–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst R.K., Bray,M., Rekosh,D. and Hammarkskjöld,M.L. (1997) Secondary structure and mutational analysis of the Mason–Pfizer monkey virus RNA constitutive transport element. RNA, 3, 210–222. [PMC free article] [PubMed] [Google Scholar]
- 30.Grüter P., Tabernero,C., Kobbe,C.V., Schmitt,C., Saaverdra,C., Bachi,A., Wilm,M., Felber,B.K. and Izaurralde,E. (1998) TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell, 1, 649–659. [DOI] [PubMed] [Google Scholar]
- 31.Liker E., Fernandez,E., Izurralde,E. and Conti,E. (2000) The structure of the mRNA export factor TAP reveals a cis arrangement of a non-canonical RNP domain and an LRR domain. EMBO J., 21, 5587–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho D.N., Coburn,G.A., Kang,Y., Cullen,B.R. and Georgiadis,M.M. (2002) The crystal structure and mutational analysis of a novel RNA-binding domain found in the human Tap nuclear mRNA export factor. Proc. Natl Acad. Sci. USA, 99, 1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myöhänen S. and Baylin,S.B. (2001) Sequence-specific DNA binding activity of RNA helicase A to the p16INK4a promoter. J. Biol. Chem., 276, 1634–1642. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima T., Uchida,C., Anderson,S.F., Parvin,J.D. and Montminy,M. (1997) RNA helicase A mediates association of CBP with RNA polymerase II. Cell, 90, 1107–1112. [DOI] [PubMed] [Google Scholar]
- 35.Aratani S., Fujii,R., Oishi,T., Fujita,H., Amano,T., Ohshima,T., Hagiwara,M., Fukamizu,A. and Nakajima,T. (2001) Dual roles of RNA helicase A in CREB-dependent transcription. Mol. Cell. Biol., 21, 4460–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang H. and Wong-Staal,F. (2000) Specific interaction between RNA helicase A and Tap, two cellular proteins that bind to the constitutive transport element of type D retrovirus. J. Biol. Chem., 275, 32694–32700. [DOI] [PubMed] [Google Scholar]
- 37.Braun I., Rohrbach,E., Schmitt,C. and Izurralde,E. (1999) TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J., 18, 1953–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manche I., Green,S.R., Schmedt,C. and Mathews,M.B. (1992) Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol., 12, 5238–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards J.P., Bächinger,H.P., Goodman,R.H. and Bernnan,R.G. (1996) Analysis of the structural properties of cAMP-responsive element-binding protein (CREB) and phosphorylated CREB. J. Biol. Chem., 271, 13716–13723. [DOI] [PubMed] [Google Scholar]
- 40.Rost B., Sander,C. and Schneider,R. (1994) PHD—an automatic mail server for protein secondary structure prediction. Comput. Appl. Biosci., 10, 53–60. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Romero,P., Rani,M., Dunker,A.K. and Obradovic,Z. (1999) Predicting protein disorder for N-, C- and internal regions. Genome Inform. Ser. Workshop Genome Inform., 10, 30–40. [PubMed] [Google Scholar]
- 42.Baus A., Dong,B., Krainer,A.R. and Howe,C.C. (1997) The intracisternal A-particle proximal enhancer-binding protein activates transcription and is identical to the RNA- and DNA-binding protein p54nrb/NonO. Mol. Cell. Biol., 17, 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kao P.N., Chen,L., Brock,G., Ng,J., Kenny,J., Smith,A.J. and Corthesy,B. (1994) Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J. Biol. Chem., 269, 20691–20699. [PubMed] [Google Scholar]
- 44.Corthesy B. and Kao,P.N. (1994) Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J. Biol. Chem., 269, 20682–20690. [PubMed] [Google Scholar]
- 45.Satoh M., Shaheen,V.M., Kao,P.N., Okano,T., Shaw,M., Yoshida,H., Richards,H.B. and Reeves,W.H. (1999) Autoantibodies define a family of proteins with conserved double-stranded RNA-binding domains as well as DNA binding activity. J. Biol. Chem., 274, 34598–34604. [DOI] [PubMed] [Google Scholar]
- 46.Balandina A., Kamashev,D. and Rouviere-Yaniv J. (2002) The bacterial histone-like protein HU specifically recognizes similar structures in all nucleic acids. J. Biol. Chem., 277, 27622–27628. [DOI] [PubMed] [Google Scholar]
- 47.Connolly K.M., Wojciak,J.M. and Clubb,R.T. (1998) Site-specific DNA binding using a variation of the double stranded RNA binding motif. Nature Struct. Biol., 5, 546–550. [DOI] [PubMed] [Google Scholar]
- 48.Tompa P. (2002) Intrinsically unstructured protein. Trends Biochem. Sci., 27, 527–533. [DOI] [PubMed] [Google Scholar]
- 49.Romano P.R., Green,S.R., Barber,G.N., Mathews,M.B. and Hinnebusch,A.G. (1995) Structural requirements for double-stranded RNA binding, dimerization and activation of the human eIF-2 alpha kinase DAI in Saccharomyces cerevisiae. Mol. Cell. Biol., 15, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmedt C., Green,S.R., Manche,L., Taylor,D.R., Ma,Y. and Mathews,M.B. (1995) Functional characterization of the RNA-binding domain and motif of the double-stranded RNA-dependent protein kinase DAI (PKR). J. Mol. Biol., 249, 29–44. [DOI] [PubMed] [Google Scholar]
- 51.McCormack S.J., Ortega,L.G., Doohan,J.P. and Samuel,C.E. (1994) Mechanism of interferon action motif I of the interferon-induced, RNA-dependent protein kinase (PKR) is sufficient to mediate RNA-binding activity. Virology, 198, 92–99. [DOI] [PubMed] [Google Scholar]
- 52.McMillan N.A., Carpick,B.W., Hollis,B., Toone,W.M., Zamanian-Daryoush,M. and Williams,B.R. (1995) Mutational analysis of the double-stranded RNA (dsRNA) binding domain of the dsRNA-activated protein kinase, PKR. J. Biol. Chem., 270, 2601–2606. [DOI] [PubMed] [Google Scholar]
- 53.Daher A., Longuet,M., Dorin,D., Bois,F., Segeral,E., Bannwarth,S., Battisti,P.L., Purcell,D.F., Benarous,R., Vaquero,C., Meurs,E.F. and Gatignol,A. (2001) Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J. Biol. Chem., 276, 33899–33905. [DOI] [PubMed] [Google Scholar]
- 54.Montminy M.R., Sevarino,K.A., Wagner,J.A., Mandel,G. and Goodman,R.H. (1986) Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Natl Acad. Sci. USA, 83, 6682–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]