Abstract
Primary cilia are sensory structures involved in morphogen signalling during development, liquid flow in the kidney, mechanosensation, sight, and smell (Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. Annu. Rev. Genomics Hum. Genet. 7:125–148; Singla, V., and J.F. Reiter. 2006. Science. 313:629–633.). Mutations that affect primary cilia are responsible for several diseases, including neural tube defects, polycystic kidney disease, retinal degeneration, and cancers (Badano et al., 2006; Singla and Reiter, 2006). Primary cilia formation and function requires tight integration of the microtubule cytoskeleton with membrane trafficking (Singla and Reiter, 2006), and this is poorly understood. We show that the Rab GTPase membrane trafficking regulators Rab8a, -17, and -23, and their cognate GTPase-activating proteins (GAPs), XM_037557, TBC1D7, and EVI5like, are involved in primary cilia formation. However, other human Rabs and GAPs are not. Additionally, Rab8a specifically interacts with cenexin/ODF2, a basal body and microtubule binding protein required for cilium biogenesis (Ishikawa, H., A. Kubo, S. Tsukita, and S. Tsukita. 2005. Nat. Cell Biol. 7:517–524), and is the sole Rab enriched at primary cilia. These findings provide a basis for understanding how specific membrane trafficking pathways cooperate with the microtubule cytoskeleton to give rise to the primary cilia.
Introduction
Primary cilia form when the basal body migrates toward the cell cortex and nucleates the formation of a ninefold array of doublet microtubules called the axoneme (Sorokin, 1968). The axoneme is closed wrapped by the plasma membrane to form a finger-like projection, to which receptors and signaling molecules involved in sensing sight, smell, and mechanical stress localize (Christensen et al., 2007). Not surprisingly, polarized trafficking is important for the delivery of components to the primary cilium, and this involves a particle known as the intraflagellar transport complex (IFT) and motor proteins of the kinesin-2 family (Scholey, 2003). Given the general role of Rabs in controlling polarized membrane trafficking and imparting identity to membrane subdomains (Zerial and McBride, 2001; Pfeffer, 2003; Behnia and Munro, 2005), it is probable that specific Rabs function in membrane transport to primary cilia and help to define this subdomain of the cell surface. Recently, two Rab-like GTPases, IFT27 and IFTA-2—Rabl4 and Rabl5 in humans—were implicated in cilium function in Chlamydomonas reinhardtii and Caenorhabditis elegans, respectively (Schafer et al., 2006; Qin et al., 2007). IFT27 has been implicated in membrane trafficking and in signaling and cell cycle control events associated with cilia formation (Qin et al., 2007), but although IFTA-2 has been implicated in cilium function, it is not required for their formation (Schafer et al., 2006). However, both Rabl4 and Rabl5 lack the C-terminal prenylation motifs that are a signature of Rabs required for their targeting to specific membrane surfaces. Therefore, although Rabl4 and Rabl5 are important factors at cilia, their function is likely to be different than that of the canonical Rab GTPases in mammalian cells. Arl GTPases have also been implicated in primary cilia function. Arl6 is mutated in Biedl-Bardet syndrome 3 (Chiang et al., 2004), and mutations in ARL13b result in truncated primary cilia defective for Hedgehog signaling (Caspary et al., 2007). Exactly how they function is unclear, but by analogy with other Arl family members, such as Arl2 and Arl3, they may be important for controlling microtubule function at cilia (Grayson et al., 2002; Zhou et al., 2006). To understand how specific membrane trafficking and tethering events contribute to the formation of cilia, we have investigated the requirement for specific Rabs and their GTPase-activating protein (GAP) regulators in primary cilium formation.
Results and discussion
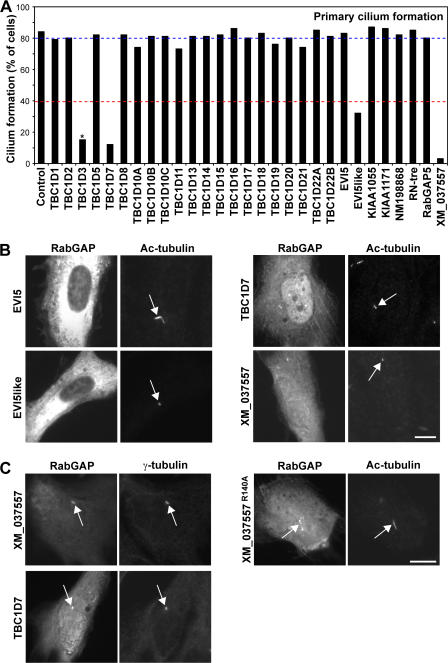
To find Rabs involved in primary cilium formation, the 39 predicted human RabGAPs were tested for their ability to prevent primary cilium formation in telomerase-immortalized retinal pigmented epithelial (hTERT-RPE1) cells (Fig. 1 A and Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200703047/DC1). This revealed that cells expressing TBC1D7, EVI5like, and XM_037557 (available from GenBank/EMBL/DDBJ under this accession no.) were compromised in their ability to form primary cilia (Fig. 1, A and B). Further support for a role of TBC1D7 and XM_037557 at primary cilia comes from the observation that they overlap with γ-tubulin to the basal body and that catalytically inactivate XM_037557 is present on the cilia (Fig. 1 C). Other GAPs either had no effect on primary cilia or, in the case of TBC1D3, caused a reduction in primary cilia accompanied with increased levels of cell death, and this is therefore unlikely to represent a specific effect (Fig. 1 A). Notably, GAPs that block Rab1-dependent secretion or Rab5-dependent endocytosis, TBC1D20 and RabGAP5 (Haas et al., 2005; unpublished data), respectively, did not have any effect on primary cilium formation (Fig. 1). General perturbation of membrane trafficking is therefore unlikely to explain the effects of TBC1D7, EVI5like, and XM_037557 on primary cilia formation. EVI5like, TBC1D7, and XM_037557 therefore represent good candidates for GAPs controlling specific Rabs involved in primary cilium formation.
Figure 1.
A subset of RabGAPs can block primary cilium formation. (A) hTERT-RPE1 cells expressing human GFP-RabGAPs were induced to form primary cilia by serum starvation and then stained for acetylated tubulin (Ac-tubulin) as a marker for primary cilia. Primary cilium formation was counted (n = 100) and is plotted for a representative series of experiments in the bar graph. The blue line marks the mean extent of cilium formation, and the red line is the 40% cutoff used to assign positive GAPs. TBC1D3 (asterisk) caused reduced cell viability and increased levels of apoptosis; TBC1D12, RUTBC1, RUTBC2, USP6, AK074305, and KIAA0882 gave similar effects and are not shown. (B) Images showing the effects of expressing EVI5like, TBC1D7, and XM_037557 on primary cilia formation. Note the lack of a primary cilium and only residual basal body staining (arrows). EVI5 is shown as a negative control where primary cilium formation is normal (arrow). (C) hTERT-RPE1 cells expressing human GFP-tagged TBC1D7, XM_037557, or the inactive XM_037557R140A mutant were induced to form primary cilia by serum starvation and then stained for γ-tubulin as a marker for the basal body or acetylated tubulin as a marker for the cilium (arrows). DNA was stained with DAPI. Bars, 10 μm.
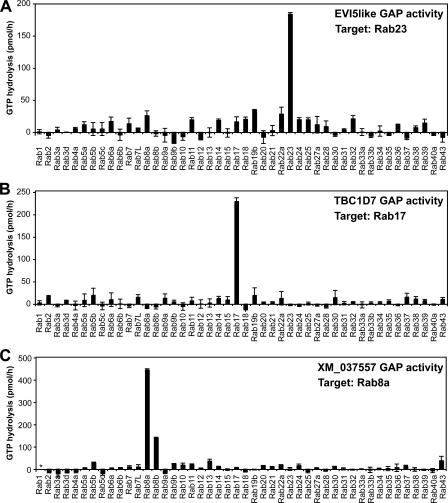
Strikingly, these GAPs showed great specificity toward single Rabs when tested in biochemical assays (Fig. 2). This approach showed that EVI5like acts on Rab23, whereas XM_037557 acts on Rab8a, and TBC1D7 acts on Rab17 (Fig. 2). Consistent with these biochemical data and the effects of GAP expression (Figs. 1 and 2), dominant-negative forms of Rab8a, -17, and -23, but not the other Rabs tested, including Rab8b, prevented primary cilium formation (Fig. S1 B). Intriguingly, Rab23 has previously been implicated as a downstream component in the Hedgehog signaling pathway (Eggenschwiler et al., 2001, 2006; Evans et al., 2003), components of which localize to and function at primary cilia (Corbit et al., 2005). The function of Rab23 in Hedgehog signaling may therefore be due to a previously unknown requirement in primary cilium formation (Fig. 1 A and Fig. 2 A). Rab17 has been previously reported to be induced during cell polarization and to be involved in the function of apical sorting endosomes in polarized epithelial cells (Lutcke et al., 1993; Zacchi et al., 1998). Its identification here (Fig. 2 B) may indicate that sorting to the primary cilium is analogous to apical-basolateral sorting in polarized epithelial cells (Ang et al., 2004). Further support for this proposal comes from the identification of Rab8a as the target of XM_037557 (Fig. 2 C), as Rab8 is known to be involved in polarized trafficking from recycling/sorting endosomes in epithelial cells (Ang et al., 2003, 2004).
Figure 2.
Identification of target Rabs for EVI5like, TBC1D7, and XM_037557. Biochemical GAP assays were performed using a representative set of human Rabs and the candidate RabGAPs EVI5like (A), TBC1D7 (B), and XM_037557 (C). GTP hydrolysis is plotted in pmol/h. The asterisk indicates nonspecific activation of the target Rab. Error bars indicate SD.
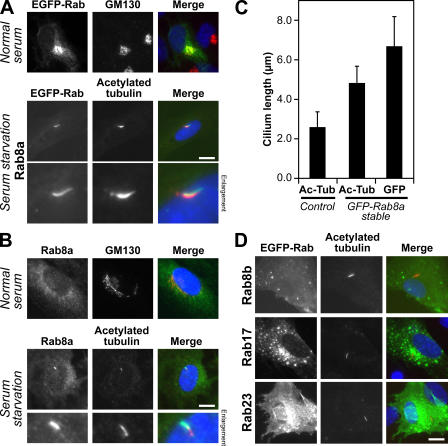
To further define the steps at which Rab8a, -17, and -23 might act, their localization was then examined in hTERT-RPE1 cells induced to form primary cilia by serum starvation. Screening the human Rabs revealed that Rab8a was the only Rab that could be detected on primary cilia when expressed as a GFP-tagged protein (Fig. 3 A and Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200703047/DC1). This localization was then confirmed using specific antibodies to Rab8a (Fig. 3 B; Hattula et al., 2006). Strikingly, cells overexpressing Rab8a typically showed significantly (P < 0.05) longer cilia than control cells, as defined by the extent of both acetylated tubulin staining and the GFP-Rab8a–positive ciliamembrane (Fig. 3 C), suggesting that it is a limiting factor for primary cilium formation. In contrast, none of the other Rabs tested (Fig. S2 A), including Rab8b (Fig. 3 D), were found at primary cilia or had any obvious effect on cilium formation. Confirming previous reports (Zacchi et al., 1998; Evans et al., 2003), Rab23 was found predominantly at the plasma membrane (Fig. 3 D), whereas Rab17 was present in punctate structures showing partial overlap with endocytic markers (Fig. 3 D and Fig. S2 B). In addition, Rabl4 and GTPases of the Arl family implicated in microtubule and cilia formation (Chiang et al., 2004; Schafer et al., 2006; Zhou et al., 2006; Qin et al., 2007) were also absent from primary cilia (Fig. S2 A). Consistent with previous reports, the only other GTPases tested showing cilium targeting were Rabl5 and Arl13b (Fig. S2 A); however, although both are important for cilium function, neither is essential for cilium formation (Schafer et al., 2006; Caspary et al., 2007). Therefore, Rab8a is the sole Rab on primary cilia and, by analogy, with the function of other Rabs, may serve to define this membrane domain (Zerial and McBride, 2001; Pfeffer, 2003; Behnia and Munro, 2005).
Figure 3.
Rab8a is the sole Rab present on primary cilia. (A) hTERT-RPE1 cells expressing human GFP-Rab8a (green) were grown in normal serum or induced to form primary cilia by serum starvation and then stained for acetylated tubulin (red) as a marker for primary cilia. (B) hTERT-RPE1 cells grown in normal serum or induced to form primary cilia by serum starvation were stained for Rab8a (green) and acetylated tubulin (red). (C) The length of primary cilia, defined by acetylated tubulin (Ac-Tub) staining, was measured in control hTERT-RPE1 cells (2.6 ± 0.8 μm) and hTERT-RPE1 cells stably expressing GFP-tagged Rab8a (4.8 ± 0.9 μm). The length of the Rab8a-positive structure (GFP) was also measured (6.7 ± 1.5 μm). These numbers are plotted on the graph with bars to show the standard from the mean (n > 100). (D) hTERT-RPE1 cells expressing Rab8b, -17, and -23 tagged with GFP (green) were induced to form primary cilia by serum starvation and then stained for acetylated tubulin (red) as a marker for primary cilia. DNA was stained with DAPI (blue). Bars, 10 μm.
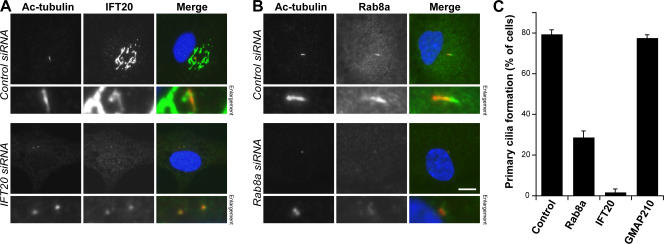
Because of its potential role in defining the membrane domain of the primary cilium, Rab8a was investigated further. To test the role of endogenous Rab8a in primary cilium formation, conditions were established for gene silencing using siRNA duplexes in hTERT-RPE1 cells using the Golgi apparatus and primary cilium protein IFT20 as a positive control (Follit et al., 2006) and the Golgi protein GMAP210 as a negative control (Fig. 4). As expected, IFT20-depleted cells were unable to form primary cilia (Fig. 4, A and C; Follit et al., 2006). Consistent with its unique localization, Rab8a-depleted cells showed strongly reduced primary cilium formation (Fig. 4, B and C). Cells depleted of the Golgi protein GMAP210 showed the same degree of primary cilium formation as control cells (Fig. 4 C). Along with the GAP-mediated Rab8a inactivation (Fig. 1), these results show that endogenous Rab8a plays an important role at the primary cilium.
Figure 4.
Rab8a is required for primary cilium formation. (A and B) hTERT-RPE1 cells treated with control, IFT20, Rab8a, or GMAP210 siRNA duplexes were induced to form primary cilia by serum starvation for 48 h. The cells were fixed and stained for acetylated tubulin (red) as a marker for primary cilia and IFT20 or Rab8a (green). DNA was stained with DAPI (blue). Bar, 10 μm. (C) The number of cells forming primary cilia was counted in cells treated as indicated in the figure. The results are plotted as a bar graph, and the standard error is shown (n = 3; 100 cells per condition).
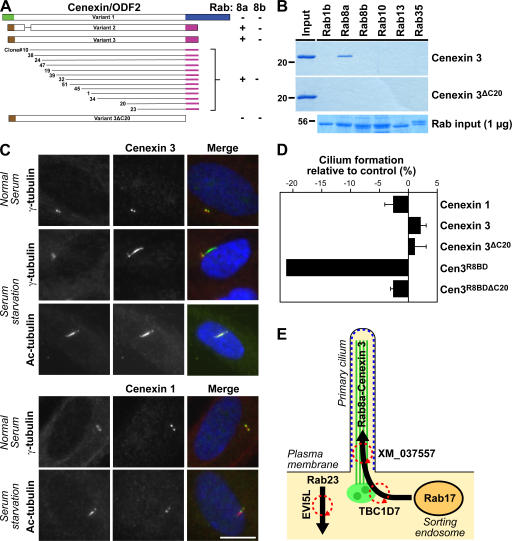
In general, Rabs are thought to function by promoting tethering interactions between membranes, and between membranes and the cytoskeleton (Behnia and Munro, 2005). Although several effector proteins for Rab8 have been reported in the literature, they are mainly associated with actin function, and none of them provide an obvious link between microtubule function and membrane traffic (Sahlender et al., 2005; Hattula et al., 2006). Furthermore, it is not known if they show any specificity for Rab8a or -8b. We reasoned that because of its unique localization, Rab8a should have specific effector proteins at the primary cilium. We therefore performed two-hybrid screening using Rab8a and counterscreened the positive clones obtained against Rab8b using established methods (Fuchs et al., 2005). Using this approach, we found that the cenexin/ODF2 splice variant 3 (cenexin 3) but not variant 1 (cenexin 1) interacted specifically with Rab8a, whereas the previously described Rab8 effector optineurin bound to both Rab8a and -8b (Fig. 5 A and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200703047/DC1; Sahlender et al., 2005). Deletion of the last 20 amino acids of cenexin 3 abolished the interaction with Rab8a, suggesting that this region forms part of the Rab8a binding domain (Fig. 5 A and Fig. S3). Confirming these findings, the interaction of Rab8a with cenexin 3 could be reconstituted using purified proteins, and this interaction was lost when the last 20 amino acids of cenexin 3 were deleted (Fig. 5 B). Importantly, cenexin 3 did not bind to other related Rabs, including Rab8b (Fig. 5 B), and therefore has the properties expected of a Rab8a-specific effector protein.
Figure 5.
Cenexin 3 is a Rab8a effector at the primary cilium. (A) 13 overlapping fragments of cenexin 3 capable of interacting with Rab8a but not Rab8b were identified using yeast two-hybrid screening of a testis cDNA library. All contain a 20-amino-acid C-terminal region absent from cenexin 1. A schematic of the different cenexin splice variants and these clones is shown in the figure; brown, green, blue, and pink indicate the alternatively spliced regions of cenexin at the N and C termini; interactions are shown by a plus sign and a lack of interaction by a minus sign. Cenexin 3ΔC20, lacking the Rab8 binding domain, did not interact with Rab8a or -8b. These data are summarized in the figure. (B) Binding assays were performed using the Rabs indicated in the figure and the C-terminal domain of cenexin 3 (aa 397–657), or the cenexin 3 C-terminal domain lacking its last 20 amino acids (ΔC20). For cenexin, the entire bound fraction and 20% of the input material are loaded on the gels shown. For Rabs, 1 μg of the input is shown. (C) hTERT-RPE1 cells expressing human FLAG–cenexin 1 or 3 (green) were grown in normal serum or induced to form primary cilia by serum starvation and stained for γ-tubulin or acetylated tubulin (red) as a marker for primary cilia. DNA was stained with DAPI (blue). Bar, 10 μm. (D) The extent of primary cilium formation in hTERT-RPE1 cells expressing cenexin 1, cenexin 3, cenexin 3ΔC20, Cen3R8BD, and Cen3R8DBΔC20 relative to the untreated control was counted and plotted in the bar graph (n = 3; 100 cells per condition). Error bars indicate SD. (E) A model for primary cilium formation. Microtubules and the basal body are shown in green, and arrows mark the Rab-regulated membrane trafficking pathways. The cilia membrane domain defined by Rab8a is marked in blue, and circular red arrows indicate the points of GAP regulation.
Cenexin/ODF2 is a basal body protein with three splice variants, differing in their N and C termini, that function in the nucleation or anchoring of microtubules at the centriole (Ishikawa et al., 2005). In light of the finding that cenexin 3 is an effector for Rab8a, we reinvestigated cenexin 1 and 3 to determine the relationship between primary cilium targeting and their Rab8a interaction properties. As previously reported, cenexin 1, which lacks the Rab8a binding domain, was present on the centrioles in the basal body both before and after serum starvation, but not on the microtubules of the primary cilium (Fig. 5 C). Strikingly, cenexin 3, although found on the basal body before induction of cilia, like Rab8a, relocated to the primary cilium on induction by serum starvation (Fig. 5 C). Furthermore, expression of the Rab8a binding domain of cenexin 3 (Cen3R8BD) had a dominant-negative effect on primary cilium formation (Fig. 5 D), supporting the idea that the Rab8a binding properties of cenexin 3 are important for its function. In contrast, full-length cenexin 1 and 3, or cenexin 3 lacking the Rab8a binding domain, had no inhibitory effect on primary cilium formation when overexpressed (Fig. 5 D). The interaction of Rab8a with cenexin 3 may therefore provide a link between membrane trafficking and tethering reactions and microtubule function at the primary cilium.
Based on the findings presented here and observations from the literature (Zacchi et al., 1998; Eggenschwiler et al., 2001, 2006; Evans et al., 2003), we propose a working model for the function of Rab8a, -17, and -23 at primary cilia (Fig. 5 E). This model draws a parallel to the function of Rab8 in polarized epithelial cells, where it is needed for transport from a sorting endosomal compartment to the cell surface (Ang et al., 2003, 2004). At primary cilia, we propose that Rab8a defines the primary cilium membrane domain by two mechanisms: first, by controlling the delivery of material to this specific region of the plasma membrane from a Rab17-positive endosomal compartment, and second, by linking the plasma membrane with cilia microtubules through the basal body and ciliary microtubule binding protein cenexin 3. In this model, Rab23, reported to act as a downstream component in Hedgehog signaling from primary cilia (Evans et al., 2003; Eggenschwiler et al., 2001, 2006), is required for retrograde transport away from primary cilia. The identification of specific Rab GTPases acting at primary cilia and their GAP regulators provides a basis for future work on membrane trafficking at primary cilia and may also be relevant for other multiciliated cells, such as those found in lung epithelia (Sorokin, 1968). Finally, these findings may also prove useful for understanding diseases where signaling pathways associated with primary cilia have become aberrantly activated (Badano et al., 2006; Michaud and Yoder, 2006; Singla and Reiter, 2006; Christensen et al., 2007).
Materials and methods
Reagents
Rabbit antibodies to Rab8a were a gift from J. Peränen (Institute of Biotechnology, University of Helsinki, Helsinki, Finland; Hattula et al., 2006). Other antibodies were as follows: sheep anti-GM130 (Shorter et al., 1999), mouse anti-EEA1 (clone 14; Becton Dickinson); mouse anti-human transferrin receptor (CBL137; Chemicon); mouse anti–γ-tubulin (GTU88; Sigma-Aldrich); mouse anti–acetylated tubulin (6-11B-1; Sigma-Aldrich); and rabbit anti-FLAG (F7425; Sigma-Aldrich). Rabbit anti-IFT20 was raised against full-length recombinant human IFT20 expressed in bacteria and affinity purified using the same protein coupled to Affigel-15 (Bio-Rad Laboratories, Inc.). Donkey secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. The methods used for cloning and construction of EGFP-tagged human Rabs and N-terminally tagged EGFP- and Myc-tagged human RabGAPs have been described previously (Haas et al., 2005; Fuchs et al., 2007). EGFP-tagged Arl expression constructs were provided by S. Munro (Medical Research Council, Laboratory of Molecular Biology, Cambridge, UK). Cenexin/ODF2 splice variants were amplified from testis and fetal cDNA (CLONTECH Laboratories, Inc.) and subcloned into pcDNA3.1 to create an N-terminal fusion to the FLAG tag. All constructs were verified by DNA sequencing (DNA sequencing service, Max Planck Institute of Biochemistry, Martinsried, Germany).
Rab GTPase assays
The purification of GST-tagged human Rab GTPases, hexahistidine-tagged TBC domain proteins from bacteria, and RabGAP assays were performed as described previously (Haas et al., 2005). Standard assays were performed for 60 min at 37°C, using 100 pmol GST-Rab and 10 pmol hexahistidine-tagged TBC domain protein. All proteins tested corresponded to the full-length open reading frames.
Rab binding assays
For binding assays, 10 μg GST-Rab protein were bound to 25 μl of packed glutathione–Sepharose (GE Healthcare) in 1 ml total volume of PBS for 60 min at 4°C. The beads were first washed three times in 500 μl with nucleotide exchange buffer (NE100: 20 mM Hepes-NaOH, pH 7.5, 100 mM NaOAc, 10 mM EDTA, and 0.1% [vol/vol] NP-40), followed by two washes in 500 μl nucleotide loading buffer (NL100: 20 mM Hepes-NaOH, pH 7.5, 100 mM NaOAc, 0.1 mM MgCl2, and 0.1% [vol/vol] NP-40). The beads were then resuspended in 200 μl NL100, and 20 μl of 100 mM GTP and 10 μg effector protein was added. Binding was then allowed to proceed for 60 min at 4°C, rotating to mix. The beads were then washed three times with 500 μl NL100, and bound proteins were eluted by the addition of elution buffer (NE200: 20 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 20 mM EDTA, and 0.1% [vol/vol] NP-40), rotating at 4°C for 15 min. Beads were pelleted by centrifugation at 2,000 g for 1 min, and the supernatant was transferred to a fresh tube. To remove contaminating Rabs, 50 μl of packed glutathione–Sepharose was added to the eluate and incubated for 10 min at 4°C with mixing. The beads were pelleted by centrifugation at 2,000 g for 1 min, and the supernatant was transferred to a fresh tube. This procedure was repeated three times. Eluted proteins were then precipitated using trichloracetic acid and analyzed on 12% minigels stained with Coomassie brilliant blue.
Assay for primary cilium formation
Human telomerase-immortalized retinal-pigmented epithelial cells (hTERT-RPE1; CLONTECH Laboratories, Inc.) were grown at 37°C and 5% CO2 in a 1:1 mixture of DME and HAMS F12 containing 10% calf serum, 2.5 mM l-glutamine, and 1.2 g/liter sodium bicarbonate. For transfection, 0.5–1.0 × 105 cells were plated in 6-well plates. For Rabs and GAP constructs, 0.5 μg plasmid DNA were mixed with 3 μl Fugene-6 according to the manufacturer's protocol (Roche Diagnostics) and then added to 1 well of the 6-well plate. For cenexin constructs, 50 ng plasmid DNA was mixed with 1 μg pBluescript-II as a carrier to avoid aggregation problems seen with high-level expression using the standard protocol. To induce primary cilium formation, the growth medium was replaced with medium lacking serum. After 48–72 h, the cells were placed on ice for 1 h, processed for fluorescence microscopy, and analyzed as described previously (Haas et al., 2005). RNA interference was performed using a published method (Haas et al., 2005). All siRNA duplexes were obtained from Dharmacon, Inc., and the target sequences were as follows: control, CGUACGCGGAAUACUUCGA; Rab8a, CAGGAACGGUUUCGGACGA, GAAUUAAACUGCAGAUAUG, GAACAAGUGUGAUGUGAAU, GAACUGGAUUCGCAACAUU; IFT20, GGAAGAGUGCAAAGACUUU; GMAP-210, GCCAGAGACAAUCUAGCAC.
Image acquisition
Cells to be imaged were fixed in −20°C methanol for 5 min and washed three times with PBS. For EEA1 staining, cells were fixed for 20 min in 3% (wt/vol) PFA, quenched for 10 min with 50 mM ammonium chloride, and permeabilized with 0.1% (vol/vol) Triton X-100 for 5 min to allow labeling of internal cell structures. Alternatively, all solutions were made in PBS, and antibody staining was performed for 60 min using a 1,000-fold dilution of antiserum or purified antibody at a final concentration of 1 μg/ml. Secondary antibodies were conjugated to Alexa 488 or Cy3, and DNA was stained with DAPI. Coverslips were mounted in 10% (wt/vol) Moviol 4–88, 1 μg/ml DAPI, 25% (wt/vol) glycerol in PBS. Images were collected using an Axioskop 2 with a 63× Plan Apochromat oil-immersion objective of NA 1.4, standard filter sets (Carl Zeiss MicroImaging, Inc.), a 1300 × 1030 pixel cooled charge-coupled device camera (model CCD-1300-Y; Princeton Instruments) and Metavue software (Visitron Systems). Images were cropped in Photoshop 7.0 or CS2 (Adobe) without contrast or other adjustments and sized and placed using Illustrator 11.0 or CS2 (Adobe).
Online supplemental material
Fig. S1 shows the effects of RabGAP and dominant-negative Rab expression on primary cilium formation. Fig. S2 shows Rab localization to primary cilia in serum-starved hTERT-RPE1 cells. Fig. S3 gives full details of the Rab8a–cenexin 3 yeast two-hybrid interaction. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200703047/DC1.
Supplementary Material
Acknowledgments
We thank Robert Kopajtich for technical assistance. S. Yoshimura thanks Xiumin Yan and Sebastien Lavoie for discussions.
Awards to F.A. Barr from the Deutsche Forschungsgemeinschaft and the Max Planck Society support S. Yoshimura and A.K. Haas, and E. Fuchs and F.A. Barr, respectively.
Abbreviations used in this paper: GAP, GTPase-activating protein; IFT, intraflagellar transport complex.
References
- Ang, A.L., H. Folsch, U.M. Koivisto, M. Pypaert, and I. Mellman. 2003. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol. 163:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, A.L., T. Taguchi, S. Francis, H. Folsch, L.J. Murrells, M. Pypaert, G. Warren, and I. Mellman. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148. [DOI] [PubMed] [Google Scholar]
- Behnia, R., and S. Munro. 2005. Organelle identity and the signposts for membrane traffic. Nature. 438:597–604. [DOI] [PubMed] [Google Scholar]
- Caspary, T., C.E. Larkins, and K.V. Anderson. 2007. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 12:767–778. [DOI] [PubMed] [Google Scholar]
- Chiang, A.P., D. Nishimura, C. Searby, K. Elbedour, R. Carmi, A.L. Ferguson, J. Secrist, T. Braun, T. Casavant, E.M. Stone, and V.C. Sheffield. 2004. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3). Am. J. Hum. Genet. 75:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.T., L.B. Pedersen, L. Schneider, and P. Satir. 2007. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 8:97–109. [DOI] [PubMed] [Google Scholar]
- Corbit, K.C., P. Aanstad, V. Singla, A.R. Norman, D.Y. Stainier, and J.F. Reiter. 2005. Vertebrate Smoothened functions at the primary cilium. Nature. 437:1018–1021. [DOI] [PubMed] [Google Scholar]
- Dawe, H.R., H. Farr, and K. Gull. 2007. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 120:7–15. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler, J.T., E. Espinoza, and K.V. Anderson. 2001. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 412:194–198. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler, J.T., O.V. Bulgakov, J. Qin, T. Li, and K.V. Anderson. 2006. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev. Biol. 290:1–12. [DOI] [PubMed] [Google Scholar]
- Evans, T.M., C. Ferguson, B.J. Wainwright, R.G. Parton, and C. Wicking. 2003. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 4:869–884. [DOI] [PubMed] [Google Scholar]
- Follit, J.A., R.A. Tuft, K.E. Fogarty, and G.J. Pazour. 2006. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 17:3781–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E., B. Short, and F.A. Barr. 2005. Assay and properties of rab6 interaction with dynein-dynactin complexes. Methods Enzymol. 403:607–618. [DOI] [PubMed] [Google Scholar]
- Fuchs, E., A.K. Haas, R.A. Spooner, S. Yoshimura, J.M. Lord, and F.A. Barr. 2007. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J. Cell Biol. 177:1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson, C., F. Bartolini, J.P. Chapple, K.R. Willison, A. Bhamidipati, S.A. Lewis, P.J. Luthert, A.J. Hardcastle, N.J. Cowan, and M.E. Cheetham. 2002. Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum. Mol. Genet. 11:3065–3074. [DOI] [PubMed] [Google Scholar]
- Haas, A.K., E. Fuchs, R. Kopajtich, and F.A. Barr. 2005. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7:887–893. [DOI] [PubMed] [Google Scholar]
- Hattula, K., J. Furuhjelm, J. Tikkanen, K. Tanhuanpaa, P. Laakkonen, and J. Peranen. 2006. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 119:4866–4877. [DOI] [PubMed] [Google Scholar]
- Ishikawa, H., A. Kubo, S. Tsukita, and S. Tsukita. 2005. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7:517–524. [DOI] [PubMed] [Google Scholar]
- Lutcke, A., S. Jansson, R.G. Parton, P. Chavrier, A. Valencia, L.A. Huber, E. Lehtonen, and M. Zerial. 1993. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J. Cell Biol. 121:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud, E.J., and B.K. Yoder. 2006. The primary cilium in cell signaling and cancer. Cancer Res. 66:6463–6467. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. 2003. Membrane domains in the secretory and endocytic pathways. Cell. 112:507–517. [DOI] [PubMed] [Google Scholar]
- Qin, H., Z. Wang, D. Diener, and J. Rosenbaum. 2007. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol. 17:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender, D.A., R.C. Roberts, S.D. Arden, G. Spudich, M.J. Taylor, J.P. Luzio, J. Kendrick-Jones, and F. Buss. 2005. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 169:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, J.C., M.E. Winkelbauer, C.L. Williams, C.J. Haycraft, R.A. Desmond, and B.K. Yoder. 2006. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J. Cell Sci. 119:4088–4100. [DOI] [PubMed] [Google Scholar]
- Scholey, J.M. 2003. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19:423–443. [DOI] [PubMed] [Google Scholar]
- Shorter, J., R. Watson, M.E. Giannakou, M. Clarke, G. Warren, and F.A. Barr. 1999. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 18:4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla, V., and J.F. Reiter. 2006. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 313:629–633. [DOI] [PubMed] [Google Scholar]
- Sorokin, S.P. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3:207–230. [DOI] [PubMed] [Google Scholar]
- Zacchi, P., H. Stenmark, R.G. Parton, D. Orioli, F. Lim, A. Giner, I. Mellman, M. Zerial, and C. Murphy. 1998. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J. Cell Biol. 140:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]
- Zhou, C., L. Cunningham, A.I. Marcus, Y. Li, and R.A. Kahn. 2006. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol. Biol. Cell. 17:2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.