Abstract
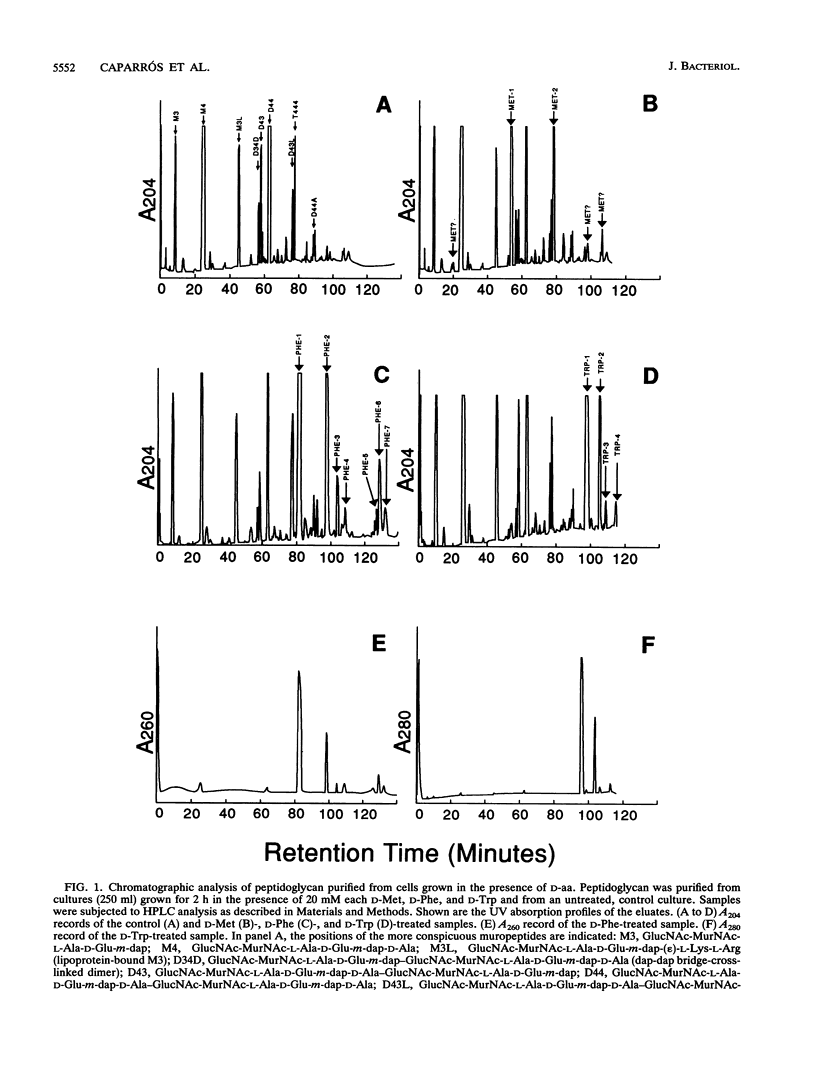
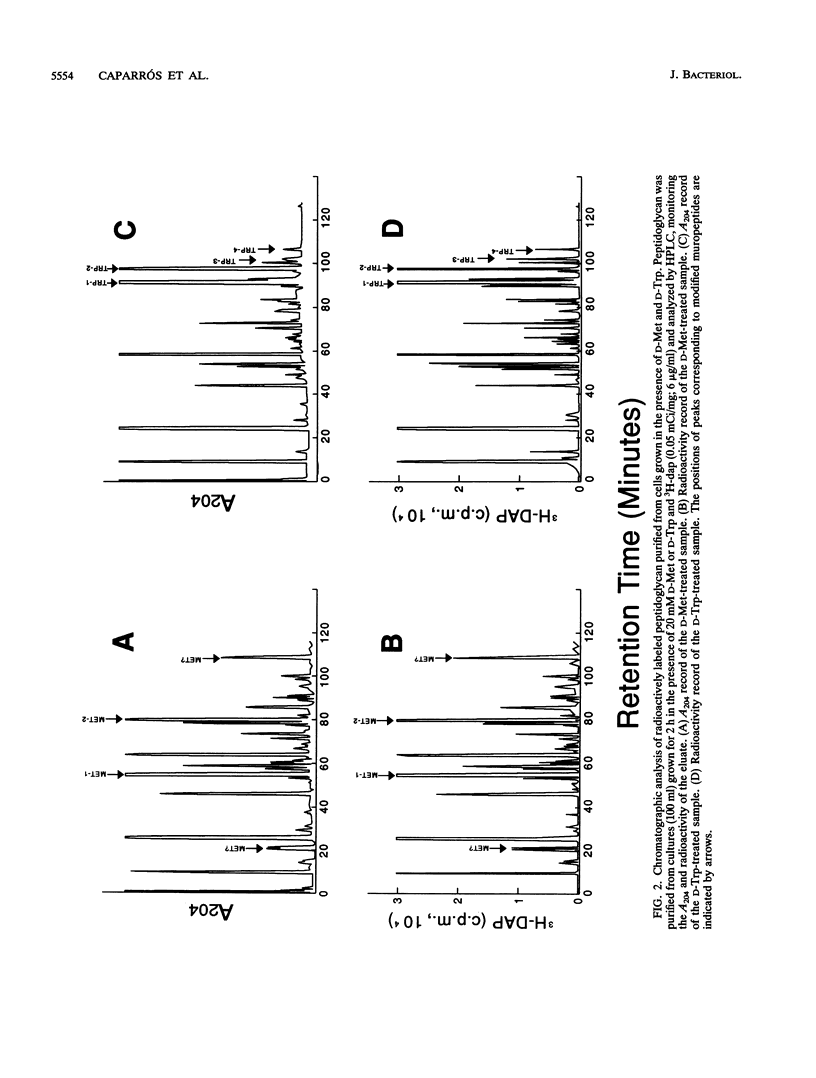
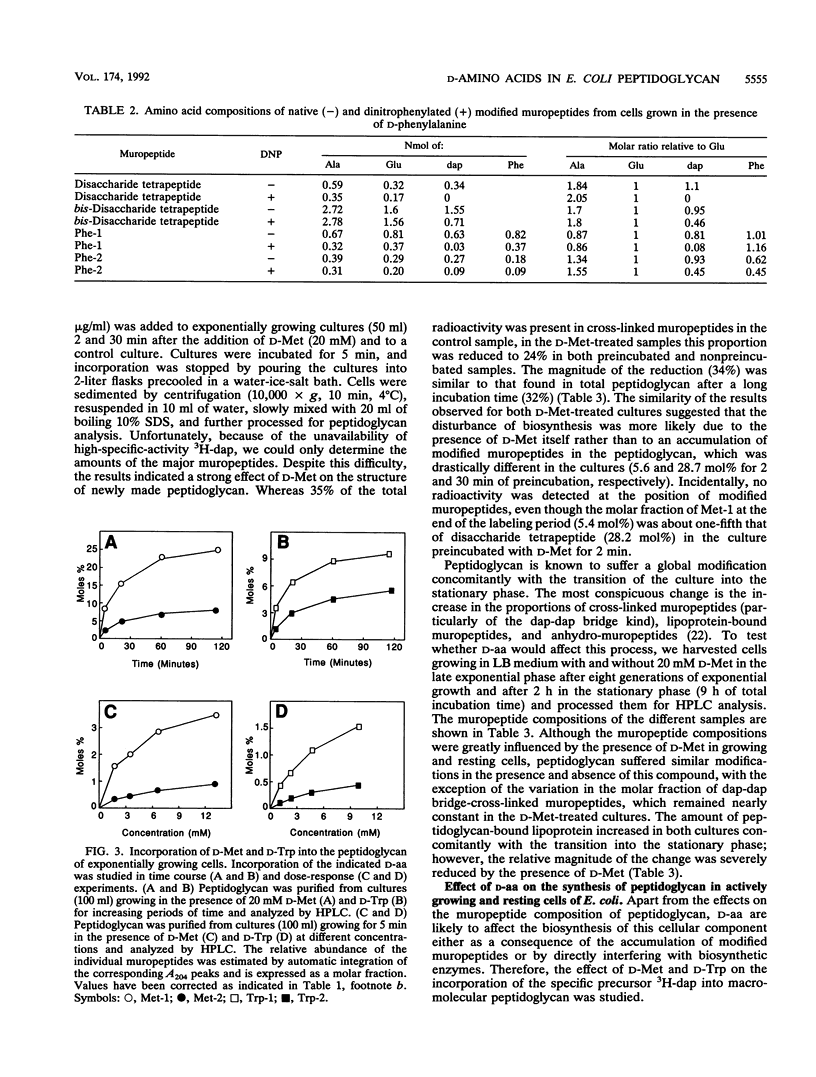
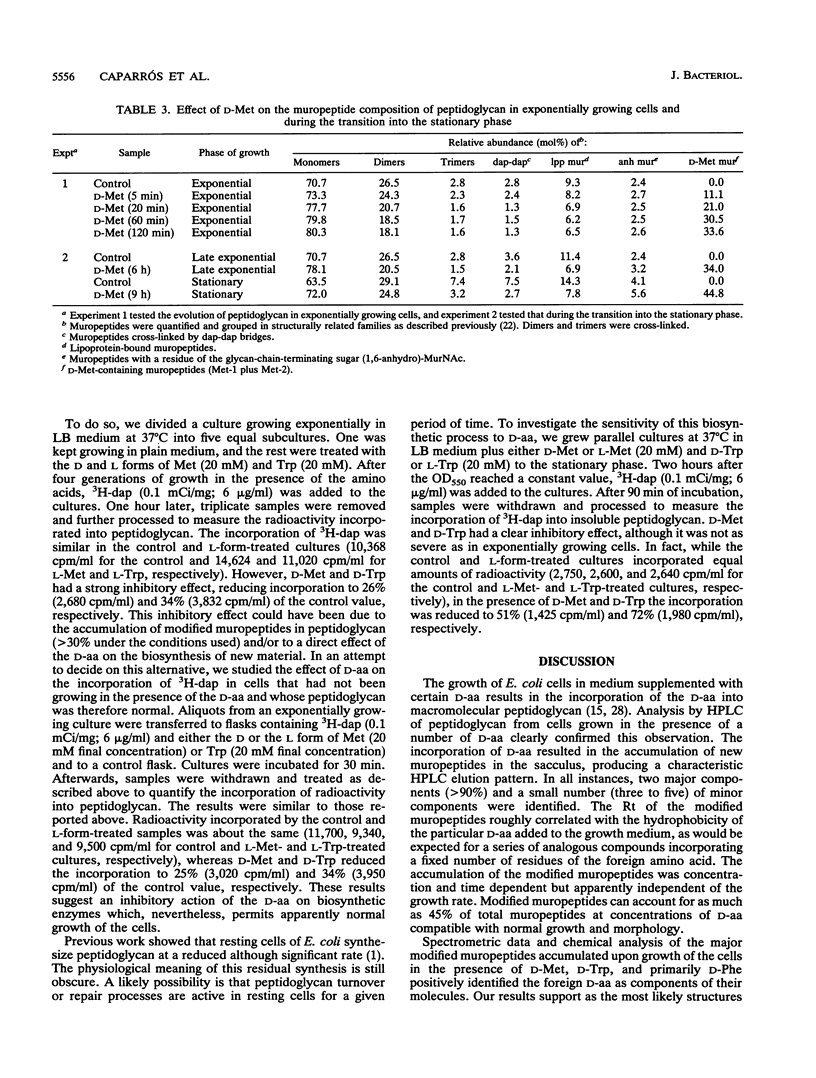
Growth of Escherichia coli in the presence of certain D-amino acids, such as D-methionine, results in the incorporation of the D-amino acid into macromolecular peptidoglycan and can be lethal at high concentrations. Previous studies suggested that incorporation was independent of the normal biosynthetic pathway. An enzymatic reaction between the D-amino acid and macromolecular peptidoglycan was proposed as the mechanism of incorporation. The application of more advanced analytical techniques, notably high-pressure liquid chromatography, revealed that the presence of a D-amino acid susceptible to incorporation induced a multiplicity of alterations in peptidoglycan metabolism. Results derived basically from the study of samples treated with D-Met, D-Trp, and D-Phe indicated that the incorporation of a D-amino acid results in the accumulation of two major new muropeptides whose general structures most likely are GlucNAc-MurNAc-L-Ala-D-Glu-m-diaminopimelic acid-D-aa and GlucNAc-MurNAc-L-Ala-D-Glu-m-diaminopimelic acid-D-Ala-GlucNAc-MurNAc-L-Ala-D-Glu-m-diaminopimelic acid-D-aa, where D-aa represents a residue of the added D-amino acid. Resting cells are proficient in the incorporation of D-amino acids and can reach peptidoglycan modification levels comparable to those in growing cells. Under our conditions, D-amino acids had no apparent effect on growth or morphology but caused a severe inhibition of peptidoglycan synthesis and cross-linking, possibly leading to a reduction in the amount of peptidoglycan per cell. The properties of the reaction support the involvement of a penicillin-insensitive LD-transpeptidase enzyme in the synthesis of modified muropeptides and a possible inhibitory action of D-amino acids on high-molecular-weight penicillin-binding proteins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasco B., Pisabarro A. G., de Pedro M. A. Peptidoglycan biosynthesis in stationary-phase cells of Escherichia coli. J Bacteriol. 1988 Nov;170(11):5224–5228. doi: 10.1128/jb.170.11.5224-5228.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Caparrós M., Torrecuadrada J. L., de Pedro M. A. Effect of D-amino acids on Escherichia coli strains with impaired penicillin-binding proteins. Res Microbiol. 1991 Feb-Apr;142(2-3):345–350. doi: 10.1016/0923-2508(91)90050-k. [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Goodell E. W. Recycling of murein by Escherichia coli. J Bacteriol. 1985 Jul;163(1):305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Cleavage and resynthesis of peptide cross bridges in Escherichia coli murein. J Bacteriol. 1983 Oct;156(1):136–140. doi: 10.1128/jb.156.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tuomanen E. I. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J Gen Microbiol. 1991 Mar;137(3):441–454. doi: 10.1099/00221287-137-3-441. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol Rev. 1988 Sep;52(3):337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J Bacteriol. 1985 Nov;164(2):861–865. doi: 10.1128/jb.164.2.861-865.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., BRADLEY D., LARK K. G. FURTHER STUDIES ON THE INCORPORATION OF D-METHIONINE INTO THE BACTERIAL CELL WALL; ITS INCORPORATION INTO THE R-LAYER AND THE STRUCTURAL CONSEQUENCES. Biochim Biophys Acta. 1963 Oct 29;78:278–288. doi: 10.1016/0006-3002(63)91638-x. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. The effects of D-amino acids on Alcaligenes fecalis. Can J Microbiol. 1959 Aug;5:369–379. doi: 10.1139/m59-046. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Cell division and peptidoglycan assembly in Escherichia coli. Mol Microbiol. 1991 Apr;5(4):791–795. doi: 10.1111/j.1365-2958.1991.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats R., de Pedro M. A. Normal growth and division of Escherichia coli with a reduced amount of murein. J Bacteriol. 1989 Jul;171(7):3740–3745. doi: 10.1128/jb.171.7.3740-3745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Tamura A., Miyata A., Takei T., Iwamatsu K., Inouye S., Matsuhashi M. Penicillin-insensitive incorporation of D-amino acids into cell wall peptidoglycan influences the amount of bound lipoprotein in Escherichia coli. J Bacteriol. 1984 Dec;160(3):889–894. doi: 10.1128/jb.160.3.889-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]


