Abstract
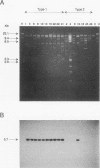
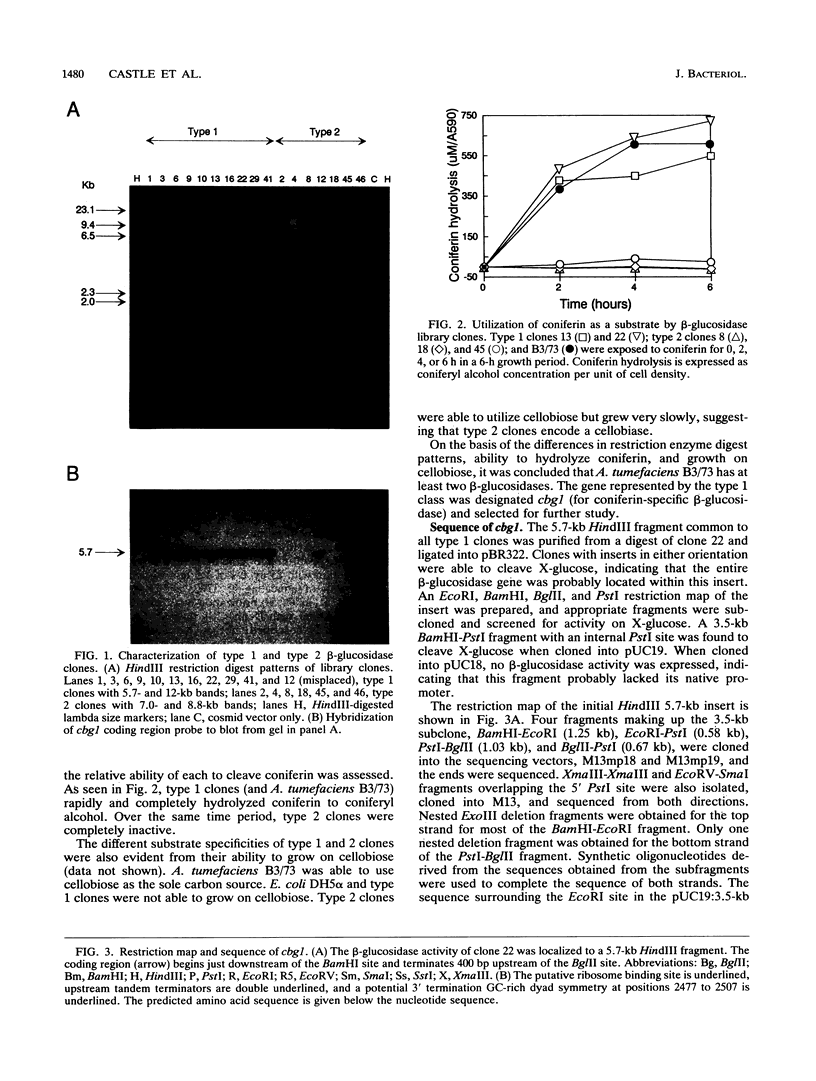
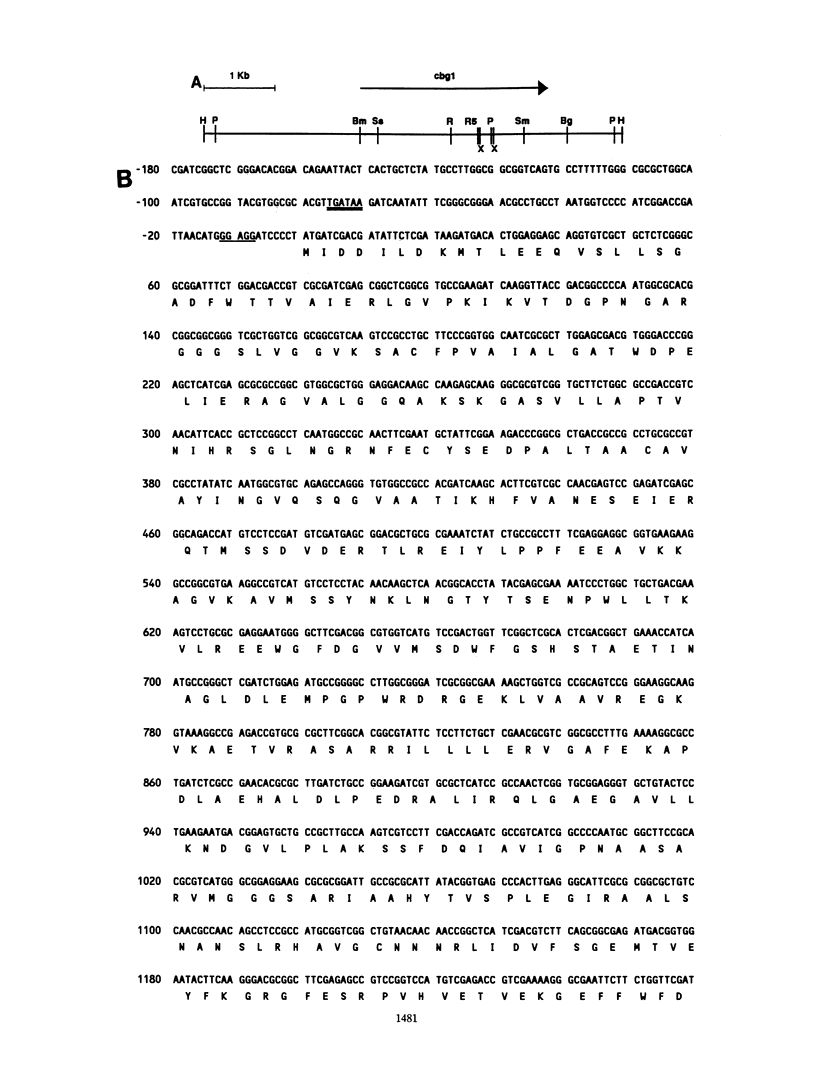
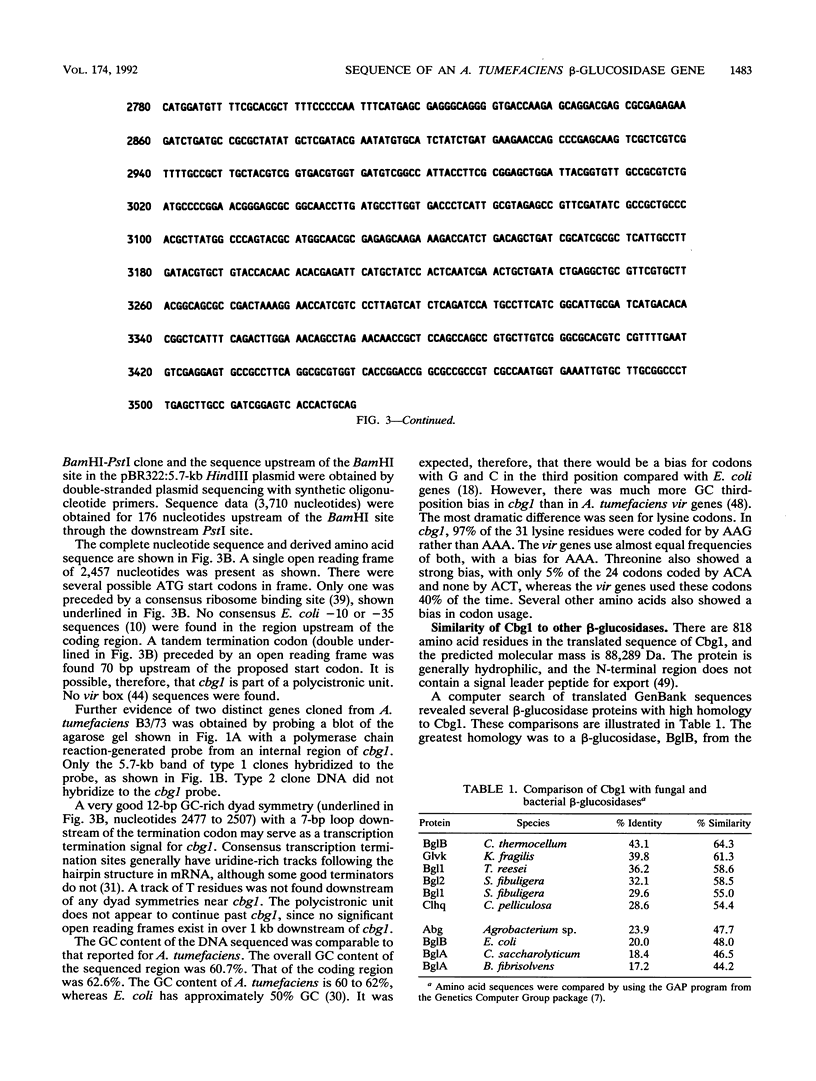
Induction of Agrobacterium tumefaciens virulence genes by plant phenolic compounds is essential for successful T-DNA transfer to a host plant. In Douglas fir needles, the major virulence region inducer is the glycoside coniferin (J. W. Morris and R. O. Morris, Proc. Natl. Acad. Sci. USA 87:3612-3618, 1990). Agrobacterium strains with high beta-glucosidase activity respond to coniferin and infect Douglas fir seedlings, whereas most strains with low beta-glucosidase activity fail to respond to coniferin and are avirulent on this host. We have cloned two beta-glucosidase genes from A. tumefaciens B3/73 and sequenced one of them, cbg1. It appears to be part of a polycistronic unit and shows a high bias for GC-rich codons. When expressed in Escherichia coli, Cbg1 beta-glucosidase hydrolyzes coniferin but not cellobiose. The 88-kDa predicted product of cbg1 is highly similar to one other bacterial beta-glucosidase and several fungal beta-glucosidases. There is little homology between Cbg1 and other bacterial beta-glucosidases, including an Agrobacterium cellobiase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett C. C., Berka R. M., Fowler T. Cloning and amplification of the gene encoding an extracellular beta-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Biotechnology (N Y) 1991 Jun;9(6):562–567. doi: 10.1038/nbt0691-562. [DOI] [PubMed] [Google Scholar]
- Bause E., Legler G. Isolation and structure of a tryptic glycopeptide from the active site of beta-glucosidase A3 from Aspergillus wentii. Biochim Biophys Acta. 1980 Dec 16;626(2):459–465. doi: 10.1016/0005-2795(80)90142-7. [DOI] [PubMed] [Google Scholar]
- Cangelosi G. A., Ankenbauer R. G., Nester E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräbnitz F., Rücknagel K. P., Seiss M., Staudenbauer W. L. Nucleotide sequence of the Clostridium thermocellum bgIB gene encoding thermostable beta-glucosidase B: homology to fungal beta-glucosidases. Mol Gen Genet. 1989 May;217(1):70–76. doi: 10.1007/BF00330944. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hess K. M., Dudley M. W., Lynn D. G., Joerger R. D., Binns A. N. Mechanism of phenolic activation of Agrobacterium virulence genes: development of a specific inhibitor of bacterial sensor/response systems. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7854–7858. doi: 10.1073/pnas.88.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. L., Cangelosi G. A., Halperin W., Nester E. W. A chromosomal Agrobacterium tumefaciens gene required for effective plant signal transduction. J Bacteriol. 1990 Apr;172(4):1814–1822. doi: 10.1128/jb.172.4.1814-1822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Heskett M. G. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 1970 Jun;60(6):969–976. doi: 10.1094/phyto-60-969. [DOI] [PubMed] [Google Scholar]
- Kohchi C., Toh-e A. Nucleotide sequence of Candida pelliculosa beta-glucosidase gene. Nucleic Acids Res. 1985 Sep 11;13(17):6273–6282. doi: 10.1093/nar/13.17.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Rumbak E., Zappe H., Thomson J. A., Woods D. R. Cloning, sequencing and analysis of expression of a Butyrivibrio fibrisolvens gene encoding a beta-glucosidase. J Gen Microbiol. 1990 Aug;136(8):1567–1576. doi: 10.1099/00221287-136-8-1567. [DOI] [PubMed] [Google Scholar]
- Love D. R., Fisher R., Bergquist P. L. Sequence structure and expression of a cloned beta-glucosidase gene from an extreme thermophile. Mol Gen Genet. 1988 Jul;213(1):84–92. doi: 10.1007/BF00333402. [DOI] [PubMed] [Google Scholar]
- Machida M., Ohtsuki I., Fukui S., Yamashita I. Nucleotide sequences of Saccharomycopsis fibuligera genes for extracellular beta-glucosidases as expressed in Saccharomyces cerevisiae. Appl Environ Microbiol. 1988 Dec;54(12):3147–3155. doi: 10.1128/aem.54.12.3147-3155.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk A. J., Schilperoort R. A., Hooykaas P. J. Specificity of signal molecules in the activation of Agrobacterium virulence gene expression. Mol Microbiol. 1989 Jul;3(7):969–977. doi: 10.1111/j.1365-2958.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk T. J., Bourret R. B., Sedee N. J., Schilperoort R. A., Hooykaas P. J. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J. 1989 Jul;8(7):1919–1925. doi: 10.1002/j.1460-2075.1989.tb03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranelli F., Barbier J. R., Dove M. J., MacKay R. M., Seligy V. L., Yaguchi M., Willick G. E. A clone coding for Schizophyllum commune beta-glucosidase: homology with a yeast beta-glucosidase. Biochem Int. 1986 Jun;12(6):905–912. [PubMed] [Google Scholar]
- Morris J. W., Morris R. O. Identification of an Agrobacterium tumefaciens virulence gene inducer from the pinaceous gymnosperm Pseudotsuga menziesii. Proc Natl Acad Sci U S A. 1990 May;87(9):3614–3618. doi: 10.1073/pnas.87.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Raynal A., Gerbaud C., Francingues M. C., Guerineau M. Sequence and transcription of the beta-glucosidase gene of Kluyveromyces fragilis cloned in Saccharomyces cerevisiae. Curr Genet. 1987;12(3):175–184. doi: 10.1007/BF00436876. [DOI] [PubMed] [Google Scholar]
- Raynal A., Guerineau M. Cloning and expression of the structural gene for beta-glucosidase of Kluyveromyces fragilis in Escherichia coli and Saccharomyces cerevisiae. Mol Gen Genet. 1984;195(1-2):108–115. doi: 10.1007/BF00332732. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz K., Toloczyki C., Rak B. Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol. 1987 Jun;169(6):2579–2590. doi: 10.1128/jb.169.6.2579-2590.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda N., Toyoda-Yamamoto A., Nagamine J., Usami S., Katayama M., Sakagami Y., Machida Y. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W., Zambryski P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 1986 Jan;83(2):379–383. doi: 10.1073/pnas.83.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Morel P., Kado C. I. Vir box sequences in Agrobacterium tumefaciens pTiC58 and A6. Nucleic Acids Res. 1988 Sep 12;16(17):8736–8736. doi: 10.1093/nar/16.17.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakarchuk W. W., Greenberg N. M., Kilburn D. G., Miller R. C., Jr, Warren R. A. Structure and transcription analysis of the gene encoding a cellobiase from Agrobacterium sp. strain ATCC 21400. J Bacteriol. 1988 Jan;170(1):301–307. doi: 10.1128/jb.170.1.301-307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Akiyoshi D. E., Regier D., Datta A., Gordon M. P., Nester E. W. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988 Apr 25;263(12):5804–5814. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Ebert P. R., Stachel S. E., Gordon M. P., Nester E. W. A gene essential for Agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8278–8282. doi: 10.1073/pnas.83.21.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]