Abstract
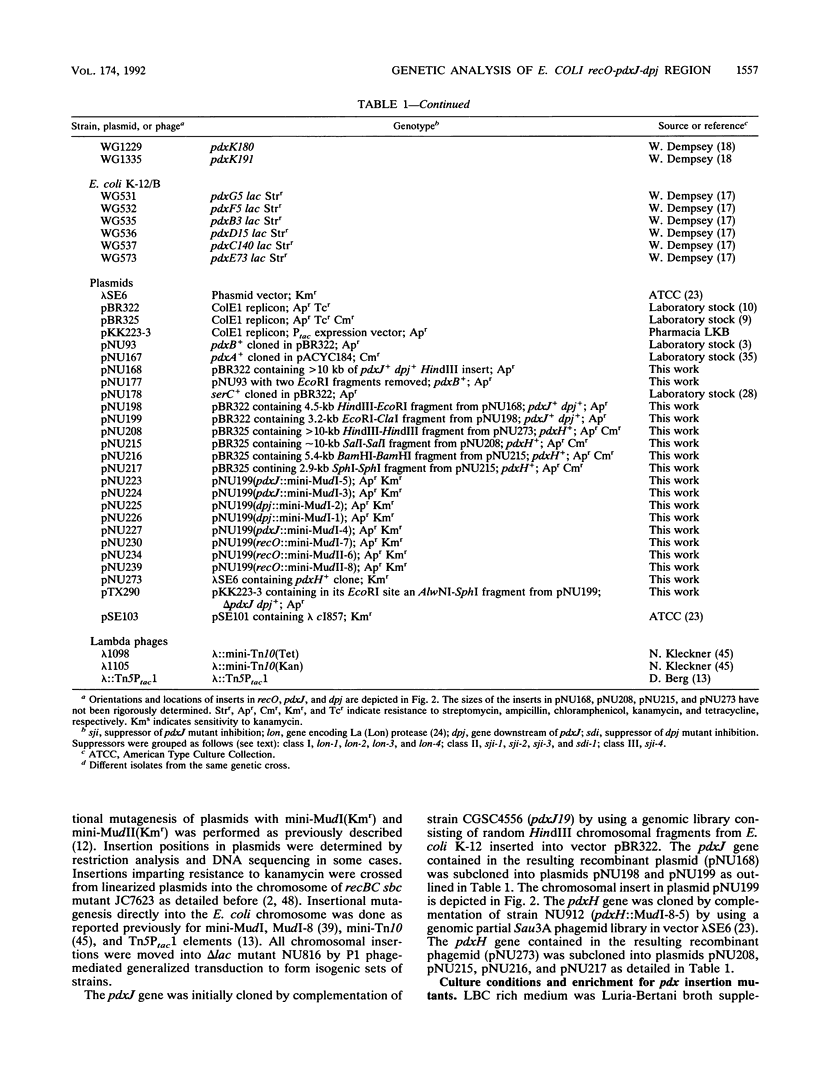
Complementation analyses using minimal recombinant clones showed that all known pdx point mutations, which cause pyridoxine (vitamin B6) or pyridoxal auxotrophy, are located in the pdxA, pdxB, serC, pdxJ, and pdxH genes. Antibiotic enrichments for chromosomal transposon mutants that require pyridoxine (vitamin B6) or pyridoxal led to the isolation of insertions in pdxA, pdxB, and pdxH but not in pdxJ. This observation suggested that pdxJ, like pdxA, pdxB, and serC, might be in a complex operon. To test this hypothesis, we constructed stable insertion mutations in and around pdxJ in plasmids and forced them into the bacterial chromosome. Physiological properties of the resulting insertion mutants were characterized, and the DNA sequence of pdxJ and adjacent regions was determined. These combined approaches led to the following conclusions: (i) pdxJ is the first gene in a two-gene operon that contains a gene, temporarily designated dpj, essential for Escherichia coli growth; (ii) expression of the rnc-era-recO and pdxJ-dpj operons can occur independently, although the pdxJ-dpj promoter may lie within recO; (iii) pdxJ encodes a 26,384-Da polypeptide whose coding region is preceded by a PDX box, and dpj probably encodes a basic, 14,052-Da polypeptide; (iv) mini-Mud insertions in dpj and pdxJ, which are polar on dpj, severely limit E. coli growth; and (v) three classes of suppressors, including mutations in lon and suppressors of lon, that allow faster growth of pdxJ::mini-Mud mutants can be isolated. A model to account for the action of dpj suppressors is presented, and aspects of this genetic analysis are related to the pyridoxal 5'-phosphate biosynthetic pathway.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnn J., March P. E., Takiff H. E., Inouye M. A GTP-binding protein of Escherichia coli has homology to yeast RAS proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8849–8853. doi: 10.1073/pnas.83.23.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Winkler M. E. An unusual genetic link between vitamin B6 biosynthesis and tRNA pseudouridine modification in Escherichia coli K-12. J Bacteriol. 1987 Mar;169(3):1071–1079. doi: 10.1128/jb.169.3.1071-1079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Winkler M. E. Structural analysis of the Escherichia coli K-12 hisT operon by using a kanamycin resistance cassette. J Bacteriol. 1987 Mar;169(3):1061–1070. doi: 10.1128/jb.169.3.1061-1070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Régnier P., Chen S. M., Nakamura Y., Grunberg-Manago M., Court D. L. Autoregulation of RNase III operon by mRNA processing. EMBO J. 1989 Nov;8(11):3401–3407. doi: 10.1002/j.1460-2075.1989.tb08504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow W. Y., Berg D. E. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6468–6472. doi: 10.1073/pnas.85.17.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Characterization of pyridoxine auxotrophs of Escherichia coli: P1 transduction. J Bacteriol. 1969 Mar;97(3):1403–1410. doi: 10.1128/jb.97.3.1403-1410.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Escherichia coli mutants tht require either pyridoxine or alanine. J Bacteriol. 1972 Sep;111(3):838–840. doi: 10.1128/jb.111.3.838-840.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Ito H. Characterization of pyridoxine auxotrophs of Escherichia coli: serine and pdxF mutants. J Bacteriol. 1970 Nov;104(2):658–667. doi: 10.1128/jb.104.2.658-667.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Pachler P. F. Isolation and characterization of pyridoxine auxotrophs of Escherichia coli. J Bacteriol. 1966 Feb;91(2):642–645. doi: 10.1128/jb.91.2.642-645.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Synthesis of Pyridoxine by a Pyridoxal Auxotroph of Escherichia coli. J Bacteriol. 1966 Aug;92(2):333–337. doi: 10.1128/jb.92.2.333-337.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Coggins J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem J. 1986 Feb 15;234(1):49–57. doi: 10.1042/bj2340049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Phasmid vectors for identification of genes by complementation of Escherichia coli mutants. J Bacteriol. 1985 May;162(2):777–783. doi: 10.1128/jb.162.2.777-783.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Genetics of proteolysis in Escherichia coli*. Annu Rev Genet. 1989;23:163–198. doi: 10.1146/annurev.ge.23.120189.001115. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H. M., Winkler M. E. Metabolic relationships between pyridoxine (vitamin B6) and serine biosynthesis in Escherichia coli K-12. J Bacteriol. 1990 Nov;172(11):6518–6528. doi: 10.1128/jb.172.11.6518-6528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Trisler P., Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985 Dec;164(3):1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill A. H., Kazarinoff M. N., Tsuge H., Horiike K., McCormick D. B. Pyridoxamine (pyridoxine) 5'-phosphate oxidase from rabbit liver. Methods Enzymol. 1979;62:568–574. doi: 10.1016/0076-6879(79)62263-2. [DOI] [PubMed] [Google Scholar]
- Morrison P. T., Lovett S. T., Gilson L. E., Kolodner R. Molecular analysis of the Escherichia coli recO gene. J Bacteriol. 1989 Jul;171(7):3641–3649. doi: 10.1128/jb.171.7.3641-3649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa B. B., Connolly D. M., Winkler M. E. Overlap between pdxA and ksgA in the complex pdxA-ksgA-apaG-apaH operon of Escherichia coli K-12. J Bacteriol. 1989 Sep;171(9):4767–4777. doi: 10.1128/jb.171.9.4767-4777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenlein P. V., Roa B. B., Winkler M. E. Divergent transcription of pdxB and homology between the pdxB and serA gene products in Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):6084–6092. doi: 10.1128/jb.171.11.6084-6092.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Baker T., Copeland T., Chen S. M., Court D. L. Locating essential Escherichia coli genes by using mini-Tn10 transposons: the pdxJ operon. J Bacteriol. 1992 Mar;174(5):1544–1553. doi: 10.1128/jb.174.5.1544-1553.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- White R. S., Dempsey W. B. Purification and properties of vitamin B6 kinase from Escherichia coli B. Biochemistry. 1970 Oct 13;9(21):4057–4064. doi: 10.1021/bi00823a005. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Kishore G. M., Snell E. E. The bacterial oxidation of vitamin B6. 4-Pyridoxic acid dehydrogenase: a membrane-bound enzyme from Pseudomonas MA-1. J Biol Chem. 1983 Aug 10;258(15):9419–9425. [PubMed] [Google Scholar]




