Abstract
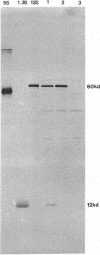
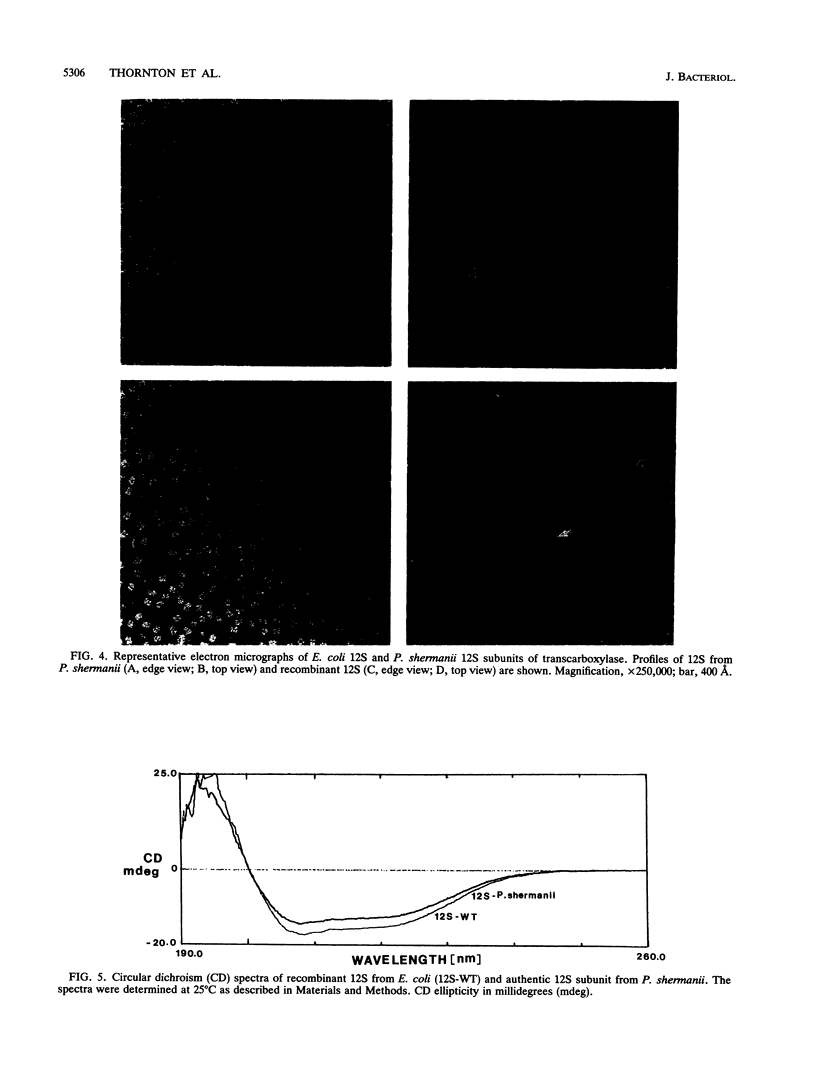
Transcarboxylase from Propionibacterium shermanii is a complex biotin-containing enzyme composed of 30 polypeptides of three different types: a hexameric central 12S subunit to which 6 outer 5S subunits are attached through 12 1.3S biotinyl subunits. The enzyme catalyzes a two-step reaction in which methylmalonyl coenzyme A and pyruvate serve as substrates to form propionyl coenzyme A (propionyl-CoA) and oxalacetate, the 12S subunit specifically catalyzing one of the two reactions. We report here the cloning, sequencing, and expression of the 12S subunit. The gene was identified by matching amino acid sequences derived from isolated authentic 12S peptides with the deduced sequence of an open reading frame present in a cloned P. shermanii genomic fragment known to contain the gene encoding the 1.3S biotinyl subunit. The cloned 12S gene encodes a protein of 604 amino acids and of M(r) 65,545. The deduced sequence shows regions of extensive homology with the beta subunit of mammalian propionyl-CoA carboxylase as well as regions of homology with acetyl-CoA carboxylase from several species. Two genomic fragments were subcloned into pUC19 in an orientation such that the 12S open reading frame could be expressed from the lac promoter of the vector. Crude extracts prepared from these cells contained an immunoreactive band on Western blots (immunoblots) which comigrated with authentic 12S. The Escherichia coli-expressed 12S was purified to apparent homogeneity by a three-step procedure and compared with authentic 12S from P. shermanii. Their quaternary structures were identical by electron microscopy, and the E. coli 12S preparation was fully active in the reactions catalyzed by this subunit. We conclude that we have cloned, sequenced, and expressed the 12S subunit which exists in a hexameric active form in E.coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M., Wood H. G. Immunochemistry of the subunits of transcarboxylase. J Biol Chem. 1976 Nov 25;251(22):7021–7034. [PubMed] [Google Scholar]
- Chuang M., Ahmad F., Jacobson B., Wood H. G. Evidence that the two partial reactions of transcarboxylation are catalyzed by two dissimilar subunits of transcarboxylase. Biochemistry. 1975 Apr 22;14(8):1611–1619. doi: 10.1021/bi00679a011. [DOI] [PubMed] [Google Scholar]
- Green N. M., Valentine R. C., Wrigley N. G., Ahmad F., Jacobson B., Wood H. G. Transcarboxylase. XI. Electron microscopy and subunit structure. J Biol Chem. 1972 Oct 10;247(19):6284–6298. [PubMed] [Google Scholar]
- Haase F. C., Beegen H., Allen S. H. Propionyl-coenzyme A carboxylase of Mycobacterium smegmatis. An electron microscopic study. Eur J Biochem. 1984 Apr 2;140(1):147–151. doi: 10.1111/j.1432-1033.1984.tb08078.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Firgaira F., Novotný J., Kalousek F., Williams K. R., Williamson C., Ohura T., Rosenberg L. E. Coding sequence of the precursor of the beta subunit of rat propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8049–8053. doi: 10.1073/pnas.83.21.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhonwah A. M., Barankiewicz T. J., Willard H. F., Mahuran D. J., Quan F., Gravel R. A. Isolation of cDNA clones coding for the alpha and beta chains of human propionyl-CoA carboxylase: chromosomal assignments and DNA polymorphisms associated with PCCA and PCCB genes. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4864–4868. doi: 10.1073/pnas.83.13.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Jan 15;267(2):855–863. [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtif V. L., Bahler C. R., Samols D. Cloning and expression of the 1.3S biotin-containing subunit of transcarboxylase. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5617–5621. doi: 10.1073/pnas.82.17.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop D. B. Transcarboxylase. VI. Kinetic analysis of the reaction mechanism. J Biol Chem. 1969 Nov 10;244(21):5808–5819. [PubMed] [Google Scholar]
- Phillips N. F., Wood H. G. Isolation of pyrophosphohistidine from pyrophosphorylated pyruvate, phosphate dikinase. Biochemistry. 1986 Apr 8;25(7):1644–1649. doi: 10.1021/bi00355a030. [DOI] [PubMed] [Google Scholar]
- Poto E. M., Wood H. G., Barden R. E., Lau E. P. Photoaffinity labeling and stoichiometry of the coenzyme A ester sites of transcarboxylase. J Biol Chem. 1978 May 10;253(9):2979–2983. [PubMed] [Google Scholar]
- Samols D., Thornton C. G., Murtif V. L., Kumar G. K., Haase F. C., Wood H. G. Evolutionary conservation among biotin enzymes. J Biol Chem. 1988 May 15;263(14):6461–6464. [PubMed] [Google Scholar]
- Shenoy B. C., Xie Y., Park V. L., Kumar G. K., Beegen H., Wood H. G., Samols D. The importance of methionine residues for the catalysis of the biotin enzyme, transcarboxylase. Analysis by site-directed mutagenesis. J Biol Chem. 1992 Sep 15;267(26):18407–18412. [PubMed] [Google Scholar]
- Skrzypczak-Jankun E., Tulinsky A., Fillers J. P., Kumar K. G., Wood H. G. Preliminary crystallographic data and quaternary structural implications of the central subunit of the multi-subunit complex transcarboxylase. J Mol Biol. 1986 Apr 5;188(3):495–498. doi: 10.1016/0022-2836(86)90172-5. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Takai T., Yokoyama C., Wada K., Tanabe T. Primary structure of chicken liver acetyl-CoA carboxylase deduced from cDNA sequence. J Biol Chem. 1988 Feb 25;263(6):2651–2657. [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Kumar G. K. Transcarboxylase: its quaternary structure and the role of the biotinyl subunit in the assembly of the enzyme and in catalysis. Ann N Y Acad Sci. 1985;447:1–22. doi: 10.1111/j.1749-6632.1985.tb18422.x. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Zwolinski G. K. Transcarboxylase: role of biotin, metals, and subunits in the reaction and its quaternary structure. CRC Crit Rev Biochem. 1976 Jun;4(1):47–122. doi: 10.3109/10409237609102558. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. Electron microscopy of the large form of transcarboxylase with six attached subunits. J Biol Chem. 1977 Feb 25;252(4):1500–1504. [PubMed] [Google Scholar]
- Zwolinski G. K., Bowien B. U., Harmon F., Wood H. G. Structure of the subunits of transcarboxylase and their relationship to the quaternary structure of transcarboxylase. Biochemistry. 1977 Oct 18;16(21):4627–4637. doi: 10.1021/bi00640a016. [DOI] [PubMed] [Google Scholar]