Abstract
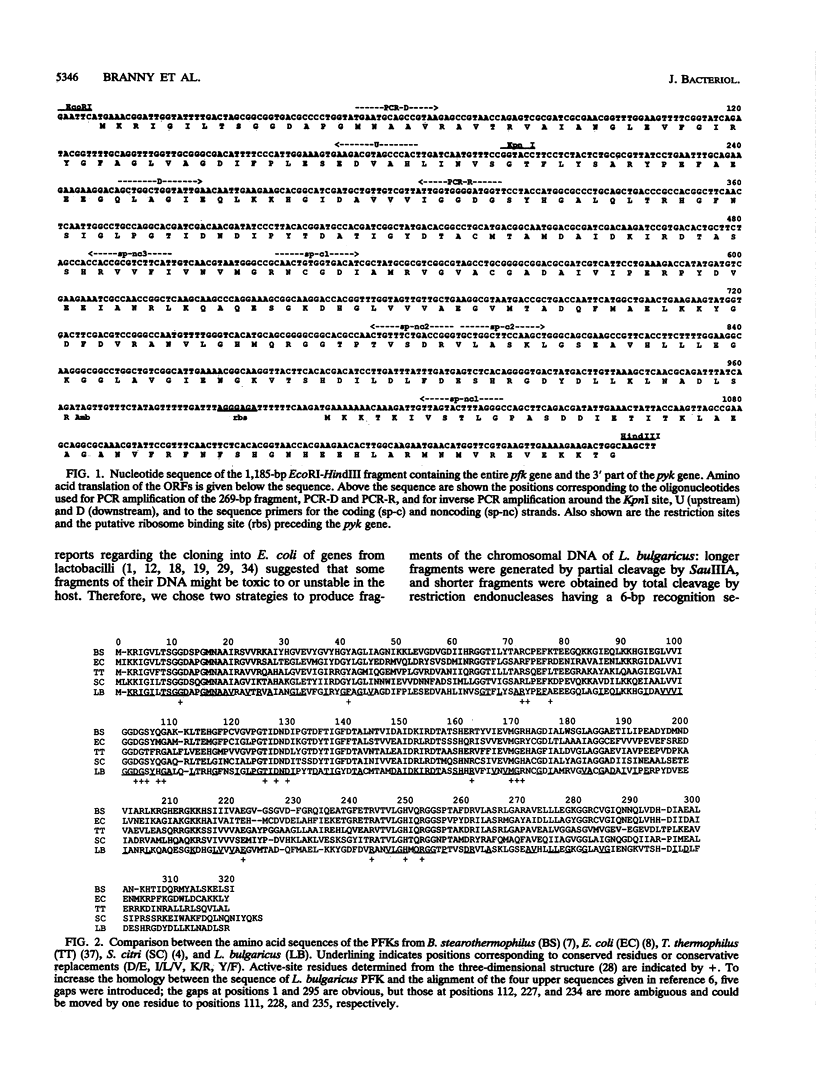
A fragment of 1,185 bp containing the gene coding for phosphofructokinase (ATP:D-fructose-6-phosphate-1-phosphotransferase; EC 2.7.1.11) in Lactobacillus bulgaricus has been cloned, sequenced, and expressed in Escherichia coli. The amino acid sequence of this enzyme was homologous to those of the ATP-dependent phosphofructokinases from E. coli, Thermus thermophilus, Spiroplasma citri, and Bacillus stearothermophilus, suggesting that these enzymes have closely related structures despite their different regulatory properties. The recombinant protein had the same structural and functional properties as did the original enzyme. The 3' end of the 1,185-bp fragment showed the presence of an open reading frame corresponding to the N-terminal amino acid sequence of the pyruvate kinase from L. bulgaricus. This gene organization, the same as that in S. citri (C. Chevalier, C. Saillard, and J. M. Bové, J. Bacteriol. 172:2693-2703, 1990) and B. stearothermophilus (D. Walker, W. N. Chia, and H. Muirhead, J. Mol. Biol. 228:265-276, 1992; H. Sakai and T. Ohta, Eur. J. Biochem. 311:851-859, 1993) but different from that in E. coli (H. W. Hellinga and P. R. Evans, Eur. J. Biochem. 149:363-373, 1985), indicated that the same transcription unit apparently contained the genes for phosphofructokinase and pyruvate kinase, the two key enzymes of glycolysis. The possibility that these genes could be transcribed at the same time suggested that in L. bulgaricus, the coordinated regulation of phosphofructokinase and pyruvate kinase occurs at the levels of both biosynthesis and enzymatic activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard N., Ferain T., Garmyn D., Hols P., Delcour J. Cloning of the D-lactate dehydrogenase gene from Lactobacillus delbrueckii subsp. bulgaricus by complementation in Escherichia coli. FEBS Lett. 1991 Sep 23;290(1-2):61–64. doi: 10.1016/0014-5793(91)81226-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Saillard C., Bové J. M. Organization and nucleotide sequences of the Spiroplasma citri genes for ribosomal protein S2, elongation factor Ts, spiralin, phosphofructokinase, pyruvate kinase, and an unidentified protein. J Bacteriol. 1990 May;172(5):2693–2703. doi: 10.1128/jb.172.5.2693-2703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F. Molecular cloning of the gene for phosphofructokinase-2 of Escherichia coli and the nature of a mutation, pfkB1, causing a high level of the enzyme. J Mol Biol. 1983 Aug 5;168(2):285–305. doi: 10.1016/s0022-2836(83)80019-9. [DOI] [PubMed] [Google Scholar]
- Fothergill-Gilmore L. A., Michels P. A. Evolution of glycolysis. Prog Biophys Mol Biol. 1993;59(2):105–235. doi: 10.1016/0079-6107(93)90001-z. [DOI] [PubMed] [Google Scholar]
- French B. A., Chang S. H. Nucleotide sequence of the phosphofructokinase gene from Bacillus stearothermophilus and comparison with the homologous Escherichia coli gene. Gene. 1987;54(1):65–71. doi: 10.1016/0378-1119(87)90348-9. [DOI] [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Mutations in the active site of Escherichia coli phosphofructokinase. Nature. 1987 Jun 4;327(6121):437–439. doi: 10.1038/327437a0. [DOI] [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Nucleotide sequence and high-level expression of the major Escherichia coli phosphofructokinase. Eur J Biochem. 1985 Jun 3;149(2):363–373. doi: 10.1111/j.1432-1033.1985.tb08934.x. [DOI] [PubMed] [Google Scholar]
- Kaluz S., Reid K. B. A novel rapid method for detection of PCR products. Nucleic Acids Res. 1991 Jul 25;19(14):4012–4012. doi: 10.1093/nar/19.14.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar S., Chuard N., Hottinger H. Cloning and overexpression of the Lactobacillus bulgaricus NAD(+)-dependent D-lactate dehydrogenase gene in Escherichia coli:purification and characterization of the recombinant enzyme. Biochem Biophys Res Commun. 1992 Jun 15;185(2):705–712. doi: 10.1016/0006-291x(92)91683-h. [DOI] [PubMed] [Google Scholar]
- Kotlarz D., Buc H. Phosphofructokinases from Escherichia coli. Methods Enzymol. 1982;90(Pt E):60–70. doi: 10.1016/s0076-6879(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bras G., Deville-Bonne D., Garel J. R. Purification and properties of the phosphofructokinase from Lactobacillus bulgaricus. A non-allosteric analog of the enzyme from Escherichia coli. Eur J Biochem. 1991 Jun 15;198(3):683–687. doi: 10.1111/j.1432-1033.1991.tb16067.x. [DOI] [PubMed] [Google Scholar]
- Le Bras G., Garel J. R. Properties of D-lactate dehydrogenase from Lactobacillus bulgaricus: a possible different evolutionary origin for the D- and L-lactate dehydrogenases. FEMS Microbiol Lett. 1991 Mar 15;63(1):89–93. doi: 10.1016/0378-1097(91)90533-g. [DOI] [PubMed] [Google Scholar]
- Lee W. T., Flynn T. G., Lyons C., Levy H. R. Cloning of the gene and amino acid sequence for glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. J Biol Chem. 1991 Jul 15;266(20):13028–13034. [PubMed] [Google Scholar]
- Lerch H. P., Blöcker H., Kallwass H., Hoppe J., Tsai H., Collins J. Cloning, sequencing and expression in Escherichia coli of the D-2-hydroxyisocaproate dehydrogenase gene of Lactobacillus casei. Gene. 1989 May 15;78(1):47–57. doi: 10.1016/0378-1119(89)90313-2. [DOI] [PubMed] [Google Scholar]
- Llanos R. M., Harris C. J., Hillier A. J., Davidson B. E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J Bacteriol. 1993 May;175(9):2541–2551. doi: 10.1128/jb.175.9.2541-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos R. M., Hillier A. J., Davidson B. E. Cloning, nucleotide sequence, expression, and chromosomal location of ldh, the gene encoding L-(+)-lactate dehydrogenase, from Lactococcus lactis. J Bacteriol. 1992 Nov;174(21):6956–6964. doi: 10.1128/jb.174.21.6956-6964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A., Morita M., Nishimura Y., Sugino Y. A rapid and highly efficient method for preparation of competent Escherichia coli cells. Nucleic Acids Res. 1990 Oct 25;18(20):6169–6169. doi: 10.1093/nar/18.20.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorman R. A., Randolph A., Kemp R. G., Heinrikson R. L. Evolution of phosphofructokinase--gene duplication and creation of new effector sites. 1984 May 31-Jun 6Nature. 309(5967):467–469. doi: 10.1038/309467a0. [DOI] [PubMed] [Google Scholar]
- Sakai H., Ohta T. Molecular cloning and nucleotide sequence of the gene for pyruvate kinase of Bacillus stearothermophilus and the production of the enzyme in Escherichia coli. Evidence that the genes for phosphofructokinase and pyruvate kinase constitute an operon. Eur J Biochem. 1993 Feb 1;211(3):851–859. doi: 10.1111/j.1432-1033.1993.tb17618.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schmidt B. F., Adams R. M., Requadt C., Power S., Mainzer S. E. Expression and nucleotide sequence of the Lactobacillus bulgaricus beta-galactosidase gene cloned in Escherichia coli. J Bacteriol. 1989 Feb;171(2):625–635. doi: 10.1128/jb.171.2.625-635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Shirakihara Y., Evans P. R. Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. J Mol Biol. 1988 Dec 20;204(4):973–994. doi: 10.1016/0022-2836(88)90056-3. [DOI] [PubMed] [Google Scholar]
- Taguchi H., Ohta T. D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, sequencing, and expression in Escherichia coli of the D-lactate dehydrogenase gene of Lactobacillus plantarum. J Biol Chem. 1991 Jul 5;266(19):12588–12594. [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Valdez B. C., French B. A., Younathan E. S., Chang S. H. Site-directed mutagenesis in Bacillus stearothermophilus fructose-6-phosphate 1-kinase. Mutation at the substrate-binding site affects allosteric behavior. J Biol Chem. 1989 Jan 5;264(1):131–135. [PubMed] [Google Scholar]
- Valentini G., Stoppini M., Speranza M. L., Malcovati M., Ferri G. Bacterial pyruvate kinases have a shorter N-terminal domain. Biol Chem Hoppe Seyler. 1991 Feb;372(2):91–93. doi: 10.1515/bchm3.1991.372.1.91. [DOI] [PubMed] [Google Scholar]
- Vanderslice P., Copeland W. C., Robertus J. D. Cloning and nucleotide sequence of wild type and a mutant histidine decarboxylase from Lactobacillus 30a. J Biol Chem. 1986 Nov 15;261(32):15186–15191. [PubMed] [Google Scholar]
- Walker D., Chia W. N., Muirhead H. Key residues in the allosteric transition of Bacillus stearothermophilus pyruvate kinase identified by site-directed mutagenesis. J Mol Biol. 1992 Nov 5;228(1):265–276. doi: 10.1016/0022-2836(92)90505-e. [DOI] [PubMed] [Google Scholar]
- Xu J., Oshima T., Yoshida M. Tetramer-dimer conversion of phosphofructokinase from Thermus thermophilus induced by its allosteric effectors. J Mol Biol. 1990 Oct 20;215(4):597–606. doi: 10.1016/S0022-2836(05)80171-8. [DOI] [PubMed] [Google Scholar]
- Xu J., Seki M., Denda K., Yoshida M. Molecular cloning of phosphofructokinase 1 gene from a thermophilic bacterium, Thermus thermophilus. Biochem Biophys Res Commun. 1991 May 15;176(3):1313–1318. doi: 10.1016/0006-291x(91)90429-b. [DOI] [PubMed] [Google Scholar]



