Abstract
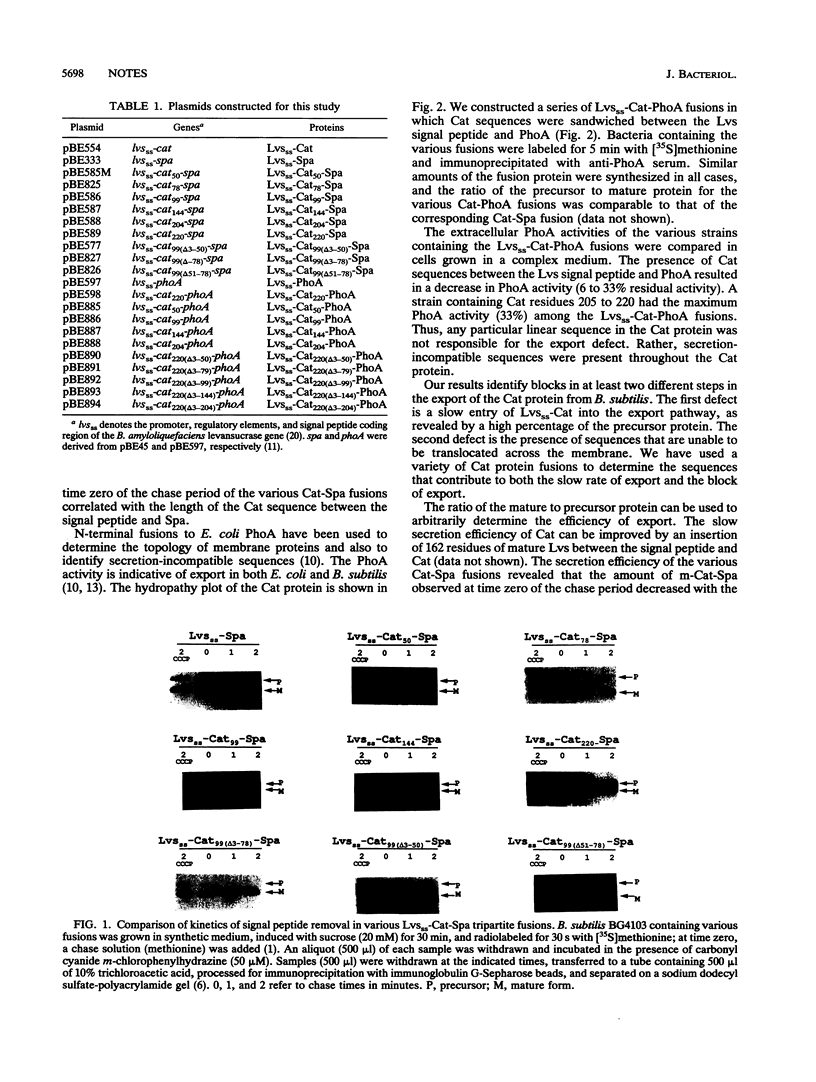
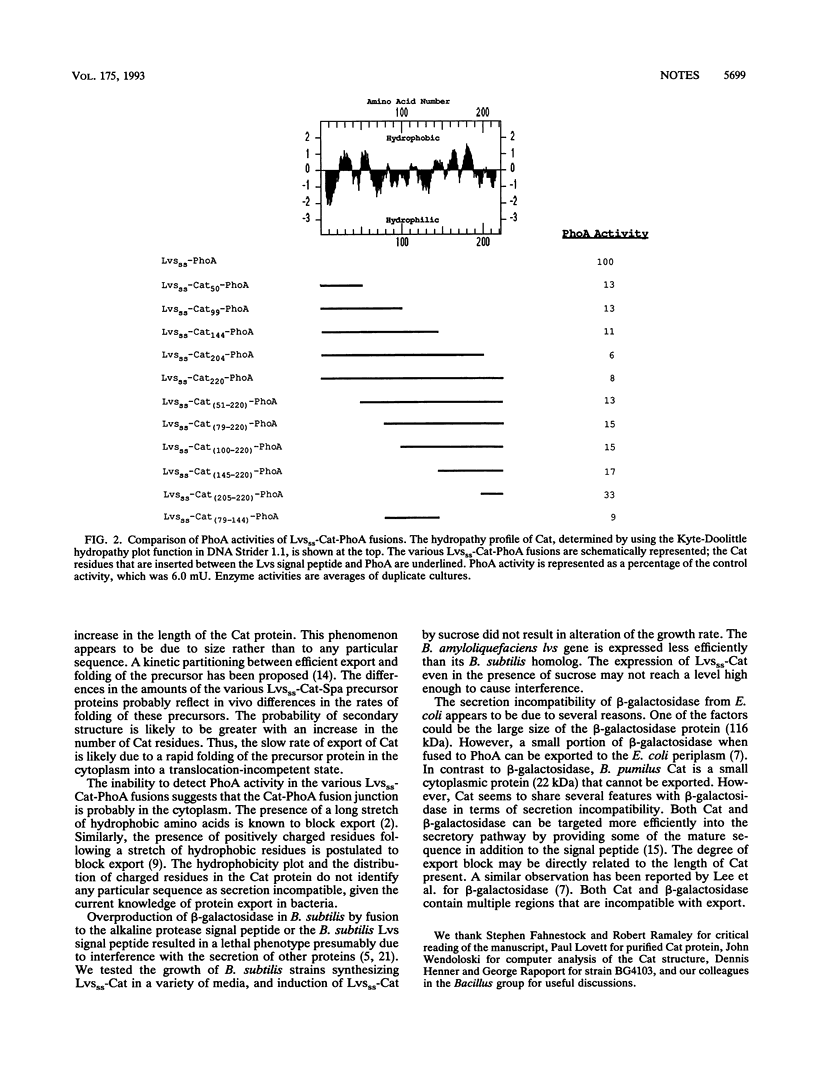
Bacillus subtilis cells expressing a hybrid protein (Lvsss-Cat) consisting of the B. amyloliquefaciens levansucrase signal peptide fused to B. pumilus chloramphenicol acetyltransferase (Cat) are unable to export Cat protein into the growth medium. A series of tripartite protein fusions was constructed by inserting various lengths of the Cat sequences between the levansucrase signal peptide and staphylococcal protein A or Escherichia coli alkaline phosphatase. Biochemical characterization of the various Cat protein fusions revealed that multiple regions in the Cat protein were causing the export defect.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchert T. V., Nagarajan V. Effect of signal sequence alterations on export of levansucrase in Bacillus subtilis. J Bacteriol. 1991 Jan;173(1):276–282. doi: 10.1128/jb.173.1.276-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. G., Model P. An artificial anchor domain: hydrophobicity suffices to stop transfer. Cell. 1985 Jun;41(2):607–614. doi: 10.1016/s0092-8674(85)80033-7. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Kramps S., Hindennach I., Henning U. Dihydrofolate reductase (mouse) and beta-galactosidase (Escherichia coli) can be translocated across the plasma membrane of E. coli. J Biol Chem. 1988 Nov 15;263(32):17084–17091. [PubMed] [Google Scholar]
- Harwood C. R., Williams D. M., Lovett P. S. Nucleotide sequence of a Bacillus pumilus gene specifying chloramphenicol acetyltransferase. Gene. 1983 Oct;24(2-3):163–169. doi: 10.1016/0378-1119(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Li P., Inouye H., Brickman E. R., Beckwith J. Genetic studies on the inability of beta-galactosidase to be translocated across the Escherichia coli cytoplasmic membrane. J Bacteriol. 1989 Sep;171(9):4609–4616. doi: 10.1128/jb.171.9.4609-4616.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A. G., Moody P. C., Shaw W. V. Structure of chloramphenicol acetyltransferase at 1.75-A resolution. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4133–4137. doi: 10.1073/pnas.85.12.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Beckwith J., Inouye H. Alteration of the amino terminus of the mature sequence of a periplasmic protein can severely affect protein export in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7685–7689. doi: 10.1073/pnas.85.20.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Nagarajan V., Albertson H., Chen M., Ribbe J. Modular expression and secretion vectors for Bacillus subtilis. Gene. 1992 May 1;114(1):121–126. doi: 10.1016/0378-1119(92)90717-4. [DOI] [PubMed] [Google Scholar]
- Nagarajan V., Borchert T. V. Levansucrase: a tool to study protein secretion in Bacillus subtilis. Res Microbiol. 1991 Sep-Oct;142(7-8):787–792. doi: 10.1016/0923-2508(91)90056-g. [DOI] [PubMed] [Google Scholar]
- Payne M. S., Jackson E. N. Use of alkaline phosphatase fusions to study protein secretion in Bacillus subtilis. J Bacteriol. 1991 Apr;173(7):2278–2282. doi: 10.1128/jb.173.7.2278-2282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. A., Bankaitis V. A., Bassford P. J., Jr Export and processing of MalE-LacZ hybrid proteins in Escherichia coli. J Bacteriol. 1984 Nov;160(2):612–617. doi: 10.1128/jb.160.2.612-617.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R. G., Harris C. R., Knowles J. R. A conservative amino acid substitution, arginine for lysine, abolishes export of a hybrid protein in Escherichia coli. Implications for the mechanism of protein secretion. J Biol Chem. 1989 Nov 25;264(33):20082–20088. [PubMed] [Google Scholar]
- Summers R. G., Knowles J. R. Illicit secretion of a cytoplasmic protein into the periplasm of Escherichia coli requires a signal peptide plus a portion of the cognate secreted protein. Demarcation of the critical region of the mature protein. J Biol Chem. 1989 Nov 25;264(33):20074–20081. [PubMed] [Google Scholar]
- Tang L. B., Lenstra R., Borchert T. V., Nagarajan V. Isolation and characterization of levansucrase-encoding gene from Bacillus amyloliquefaciens. Gene. 1990 Nov 30;96(1):89–93. doi: 10.1016/0378-1119(90)90345-r. [DOI] [PubMed] [Google Scholar]
- Zagorec M., Steinmetz M. Expression of levansucrase-beta-galactosidase hybrids inhibits secretion and is lethal in Bacillus subtilis. J Gen Microbiol. 1990 Jun;136(6):1137–1143. doi: 10.1099/00221287-136-6-1137. [DOI] [PubMed] [Google Scholar]



