Abstract
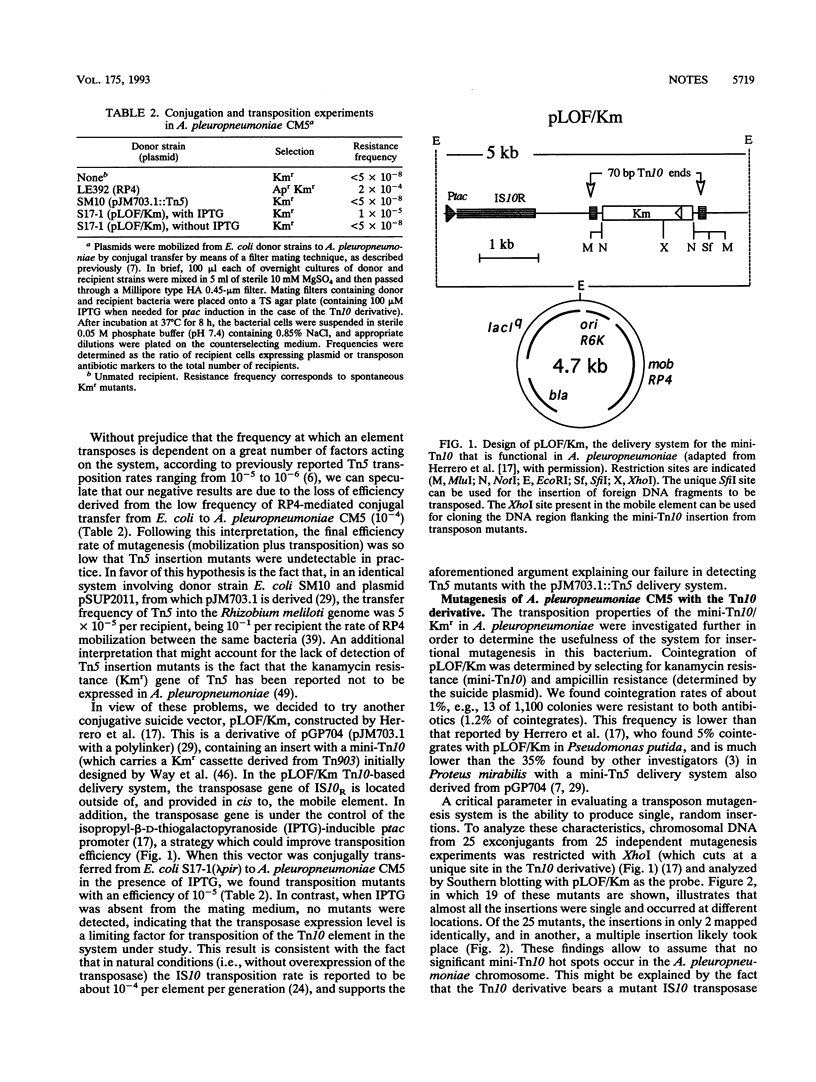
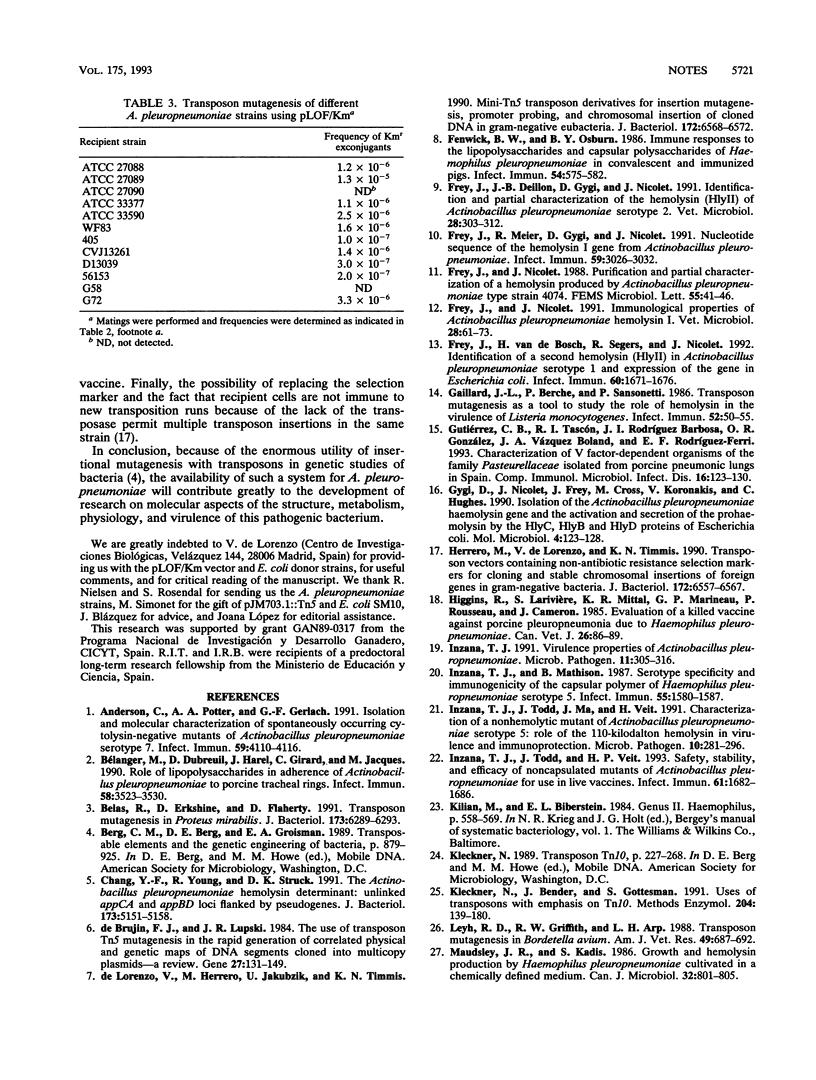
A transposon mutagenesis procedure functional in the gram-negative swine pathogen Actinobacillus pleuropneumoniae was developed for the first time. The technique involved the use of a suicide conjugative plasmid, pLOF/Km, carrying a mini-Tn10 with an isopropyl-beta-D-thiogalactopyranoside (IPTG)-inducible transposase located outside the mobile element (M. Herrero, V. de Lorenzo, and K. N. Timmis, J. Bacteriol. 172:6557-6567, 1990). The plasmid was mobilized from Escherichia coli to A. pleuropneumoniae through the RP4-mediated broad-host-range conjugal transfer functions provided by the chromosome of the donor strain. When IPTG was present in the mating medium, A. pleuropneumoniae CM5 transposon mutants were obtained at a frequency of 10(-5), while no mutants were detected in the absence of IPTG. Since the frequency of conjugal transfer of the RP4 plasmid from E. coli to A. pleuropneumoniae CM5 was found to be as low as 10(-4), the above result indicated that the expression level of the transposase was a critical factor for obtaining a workable efficiency of transposon mutagenesis. The transposon insertions occurred at random, as determined by Southern blotting of chromosomal DNA of randomly selected mutants and by the ability to generate mutants defective for the selected phenotypes. Almost all the mutants analyzed resulted from a single insertion of the Tn10 element. About 1.2% of the mutants resulted from the cointegration of pLOF/Km into the A. pleuropneumoniae chromosome. The applicability of this transposon mutagenesis system was verified on other A. pleuropneumoniae strains of different serotypes. The usefulness of this transposon mutagenesis system in genetic studies of A. pleuropneumoniae is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C., Potter A. A., Gerlach G. F. Isolation and molecular characterization of spontaneously occurring cytolysin-negative mutants of Actinobacillus pleuropneumoniae serotype 7. Infect Immun. 1991 Nov;59(11):4110–4116. doi: 10.1128/iai.59.11.4110-4116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991 Oct;173(19):6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. The Actinobacillus pleuropneumoniae hemolysin determinant: unlinked appCA and appBD loci flanked by pseudogenes. J Bacteriol. 1991 Aug;173(16):5151–5158. doi: 10.1128/jb.173.16.5151-5158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Deillon J. B., Gygi D., Nicolet J. Identification and partial characterization of the hemolysin (HlyII) of Actinobacillus pleuropneumoniae serotype 2. Vet Microbiol. 1991 Aug 15;28(3):303–312. doi: 10.1016/0378-1135(91)90085-t. [DOI] [PubMed] [Google Scholar]
- Frey J., Meier R., Gygi D., Nicolet J. Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect Immun. 1991 Sep;59(9):3026–3032. doi: 10.1128/iai.59.9.3026-3032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Immunological properties of Actinobacillus pleuropneumoniae hemolysin I. Vet Microbiol. 1991 Jun;28(1):61–73. doi: 10.1016/0378-1135(91)90099-2. [DOI] [PubMed] [Google Scholar]
- Frey J., van den Bosch H., Segers R., Nicolet J. Identification of a second hemolysin (HlyII) in Actinobacillus pleuropneumoniae serotype 1 and expression of the gene in Escherichia coli. Infect Immun. 1992 Apr;60(4):1671–1676. doi: 10.1128/iai.60.4.1671-1676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez C. B., Tascón R. I., Rodríguez Barbosa J. I., González O. R., Vázquez J. A., Rodríguez Ferri E. F. Characterization of V factor-dependent organisms of the family Pasteurellaceae isolated from porcine pneumonic lungs in Spain. Comp Immunol Microbiol Infect Dis. 1993 Apr;16(2):123–130. doi: 10.1016/0147-9571(93)90004-o. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Timmis K. N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990 Nov;172(11):6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R., Larivière S., Mittal K. R., Martineau G. P., Rousseau P., Cameron J. Evaluation of a Killed Vaccine Against Porcine Pleuropneumonia Due to Haemophilus pleuropneumoniae. Can Vet J. 1985 Feb;26(2):86–89. [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Ma J. N., Veit H. Characterization of a non-hemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb Pathog. 1991 Apr;10(4):281–296. doi: 10.1016/0882-4010(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Veit H. P. Safety, stability, and efficacy of noncapsulated mutants of Actinobacillus pleuropneumoniae for use in live vaccines. Infect Immun. 1993 May;61(5):1682–1686. doi: 10.1128/iai.61.5.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Virulence properties of Actinobacillus pleuropneumoniae. Microb Pathog. 1991 Nov;11(5):305–316. doi: 10.1016/0882-4010(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Bender J., Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- Leyh R. D., Griffith R. W., Arp L. H. Transposon mutagenesis in Bordetella avium. Am J Vet Res. 1988 May;49(5):687–692. [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S. Growth and hemolysin production by Haemophilus pleuropneumoniae cultivated in a chemically defined medium. Can J Microbiol. 1986 Oct;32(10):801–805. doi: 10.1139/m86-147. [DOI] [PubMed] [Google Scholar]
- McWhinney D. R., Chang Y. F., Young R., Struck D. K. Separable domains define target cell specificities of an RTX hemolysin from Actinobacillus pleuropneumoniae. J Bacteriol. 1992 Jan;174(1):291–297. doi: 10.1128/jb.174.1.291-297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet Scand. 1986;27(3):453–455. doi: 10.1186/BF03548158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect Immun. 1986 Dec;54(3):751–760. doi: 10.1128/iai.54.3.751-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Carpenter D. S., Mitchell W. R., Wilson M. R. Vaccination against pleuropneumonia of pigs caused by Haemophilus pleuropneumoniae. Can Vet J. 1981 Feb;22(2):34–35. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., MacInnes J. I. Characterization of an attenuated strain of Actinobacillus pleuropneumoniae, serotype 1. Am J Vet Res. 1990 May;51(5):711–717. [PubMed] [Google Scholar]
- Rosendal S., Miniats O. P., Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986 Sep;12(3):229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Smits M. A., Briaire J., Jansen R., Smith H. E., Kamp E. M., Gielkens A. L. Cytolysins of Actinobacillus pleuropneumoniae serotype 9. Infect Immun. 1991 Dec;59(12):4497–4504. doi: 10.1128/iai.59.12.4497-4504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thwaits R. N., Kadis S. Immunogenicity of Actinobacillus pleuropneumoniae outer membrane proteins and enhancement of phagocytosis by antibodies to the proteins. Infect Immun. 1991 Feb;59(2):544–549. doi: 10.1128/iai.59.2.544-549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson P. J. Haemophilus, Actinobacillus, Pasteurella: mechanisms of resistance and antibiotic therapy. Can J Vet Res. 1990 Apr;54 (Suppl):S73–S77. [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990 Nov;172(11):6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]