Abstract
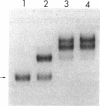
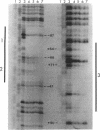
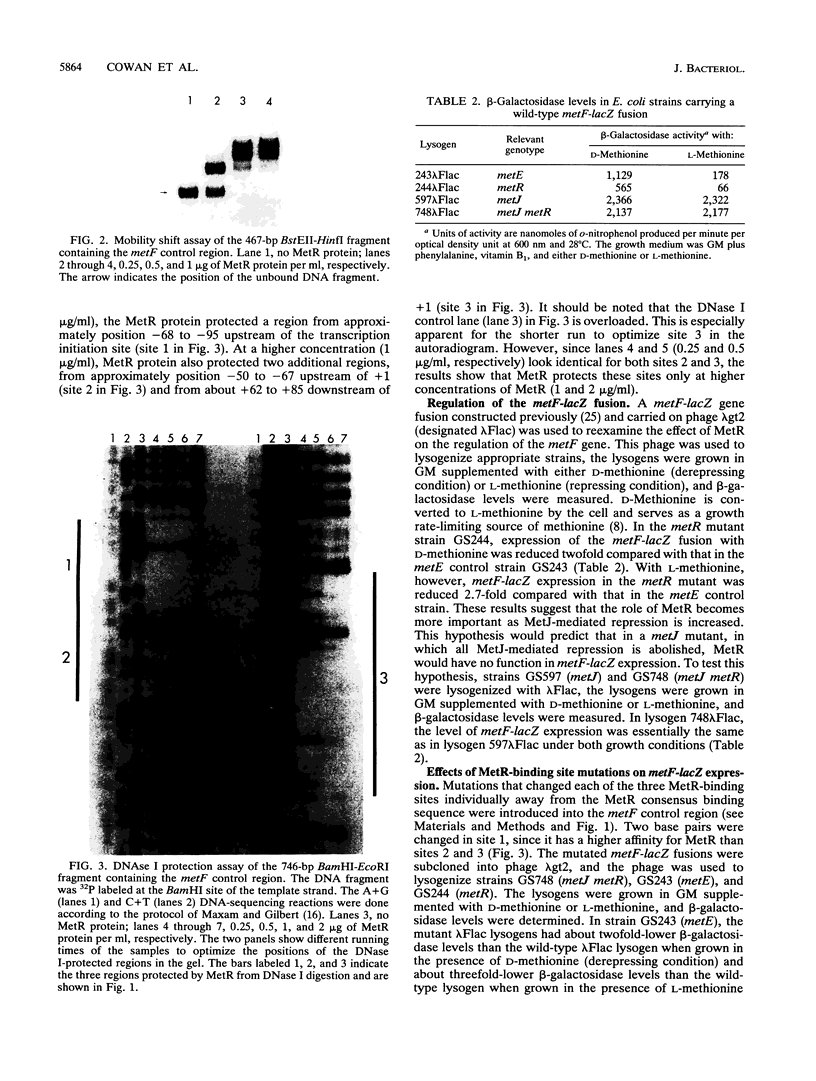
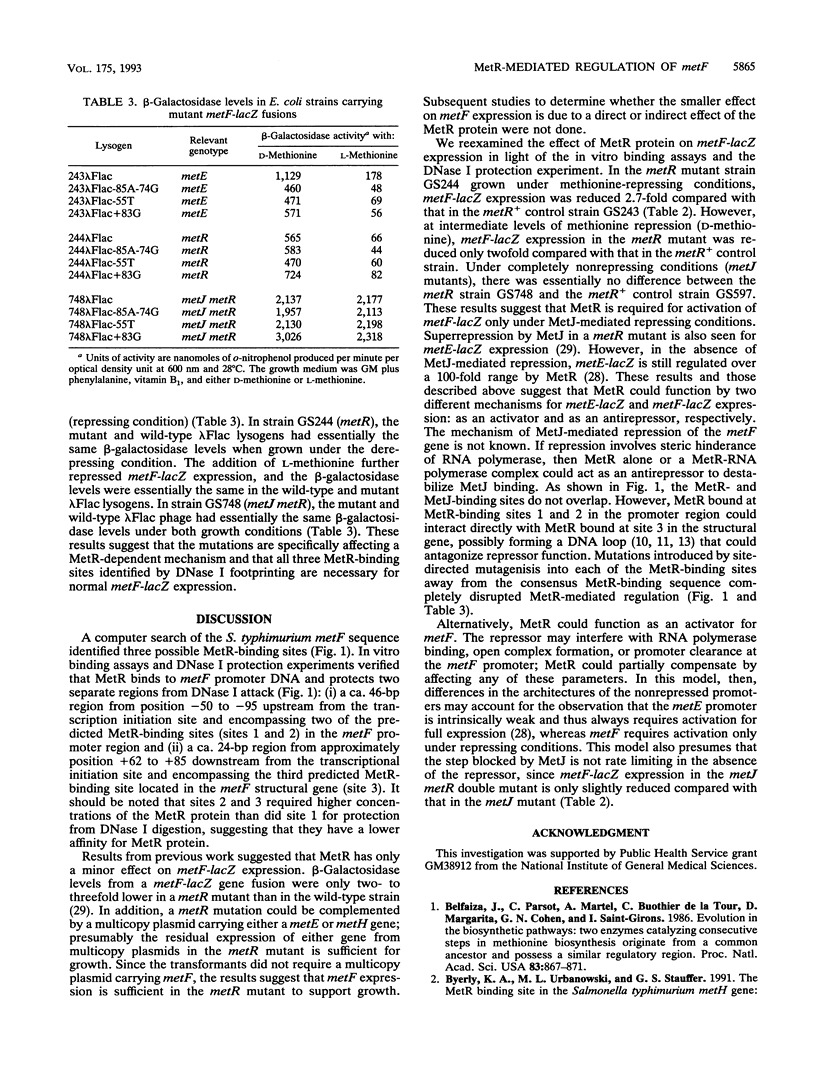
The metF gene in Escherichia coli and Salmonella typhimurium is under negative transcriptional control by the MetJ repressor. Expression of an S. typhimurium metF-lacZ gene fusion is repressed up to 10-fold by methionine addition to the growth medium in E. coli hosts encoding wild-type MetJ repressor; this repression is not seen in metJ mutants. metR mutations which eliminate the MetR activator protein result in two- to threefold-more-severe repression by the MetJ repressor. In a metJ metR double mutant, however, the level of metF-lacZ expression is the same as in a metJ mutant, suggesting that MetR antagonizes MetJ-mediated methionine repression of the metF promoter. A DNA footprint analysis showed that MetR binds to a DNA fragment carrying the metF promoter and protects two separate regions from DNase I digestion: a 46-bp region from position -50 to -95 upstream of the transcription initiation site and a 24-bp region from about position +62 to +85 downstream of the transcription initiation site and within the metF structural gene. Nucleotide changes in each of the MetR-binding sites away from the consensus sequence disrupt MetR-mediated regulation of the metF-lacZ fusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Y., Maxon M. E., Redfield B., Glass R., Brot N., Weissbach H. Methionine synthesis in Escherichia coli: effect of the MetR protein on metE and metH expression. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4407–4411. doi: 10.1073/pnas.86.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C. Methionine limitation in Escherichia coli K-12 by growth on the sulfoxides of D-methionine. J Bacteriol. 1973 Oct;116(1):230–234. doi: 10.1128/jb.116.1.230-234.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell. 1986 Mar 14;44(5):681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Huo L., Martin K. J., Schleif R. Alternative DNA loops regulate the arabinose operon in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5444–5448. doi: 10.1073/pnas.85.15.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. H., Schleif R. F. In vivo DNA loops in araCBAD: size limits and helical repeat. Proc Natl Acad Sci U S A. 1989 Jan;86(2):476–480. doi: 10.1073/pnas.86.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares R., Urbanowski M. L., Stauffer G. V. Regulation of the Salmonella typhimurium metA gene by the metR protein and homocysteine. J Bacteriol. 1992 Jan;174(2):390–397. doi: 10.1128/jb.174.2.390-397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Manfield I., Parsons I., Davidson B. E., Rafferty J. B., Somers W. S., Margarita D., Cohen G. N., Saint-Girons I., Stockley P. G. Cooperative tandem binding of met repressor of Escherichia coli. Nature. 1989 Oct 26;341(6244):711–715. doi: 10.1038/341711a0. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Parsot C., Zakin M. M., Bârzu O., Cohen G. N. Methionine biosynthesis in Enterobacteriaceae: biochemical, regulatory, and evolutionary aspects. CRC Crit Rev Biochem. 1988;23 (Suppl 1):S1–42. doi: 10.3109/10409238809083374. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Stauffer L. T. Cloning and nucleotide sequence of the Salmonella typhimurium LT2 metF gene and its homology with the corresponding sequence of Escherichia coli. Mol Gen Genet. 1988 May;212(2):246–251. doi: 10.1007/BF00334692. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Stauffer L. T. Salmonella typhimurium LT2 metF operator mutations. Mol Gen Genet. 1988 Sep;214(1):32–36. doi: 10.1007/BF00340175. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Plamann L. S., Stauffer G. V. Mutations affecting the regulation of the metB gene of Salmonella typhimurium LT2. J Bacteriol. 1987 Jan;169(1):126–130. doi: 10.1128/jb.169.1.126-130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Genetic and biochemical analysis of the MetR activator-binding site in the metE metR control region of Salmonella typhimurium. J Bacteriol. 1989 Oct;171(10):5620–5629. doi: 10.1128/jb.171.10.5620-5629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. The control region of the metH gene of Salmonella typhimurium LT2: an atypical met promoter. Gene. 1988 Dec 15;73(1):193–200. doi: 10.1016/0378-1119(88)90325-3. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer L. T., Plamann L. S., Stauffer G. V. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Apr;169(4):1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]