Abstract
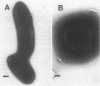
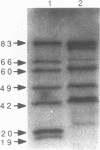
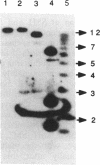
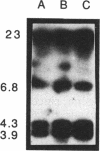
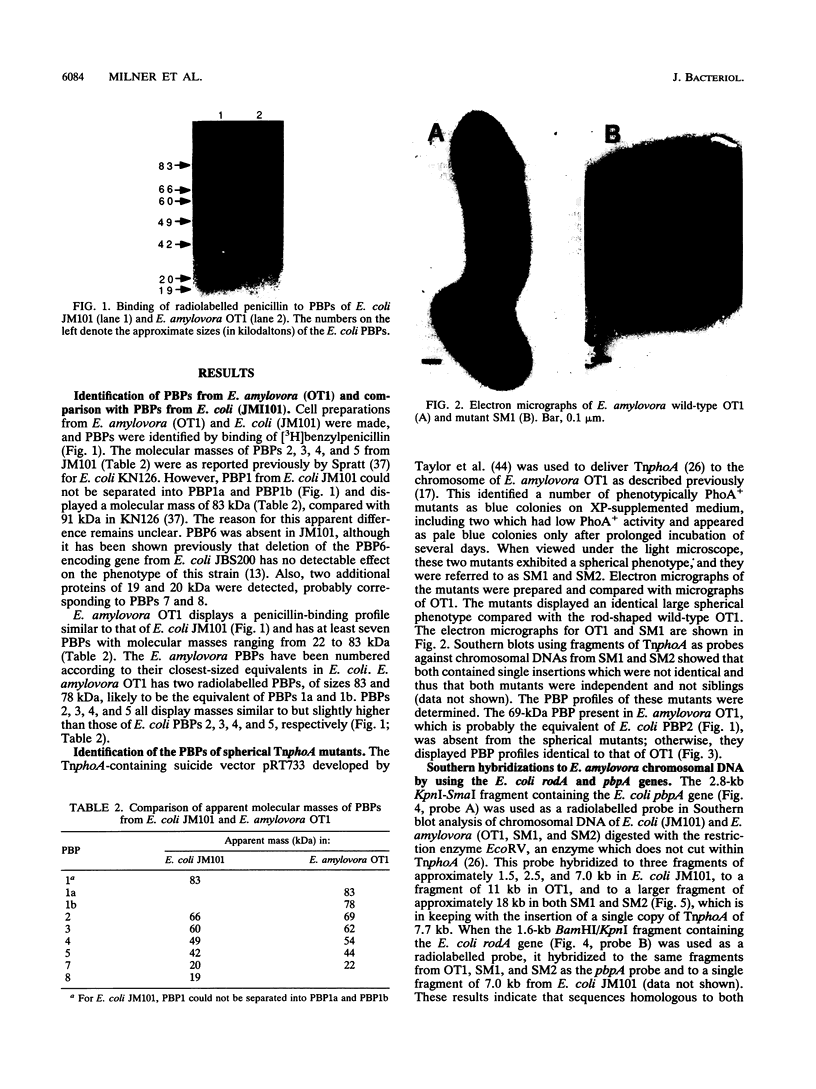
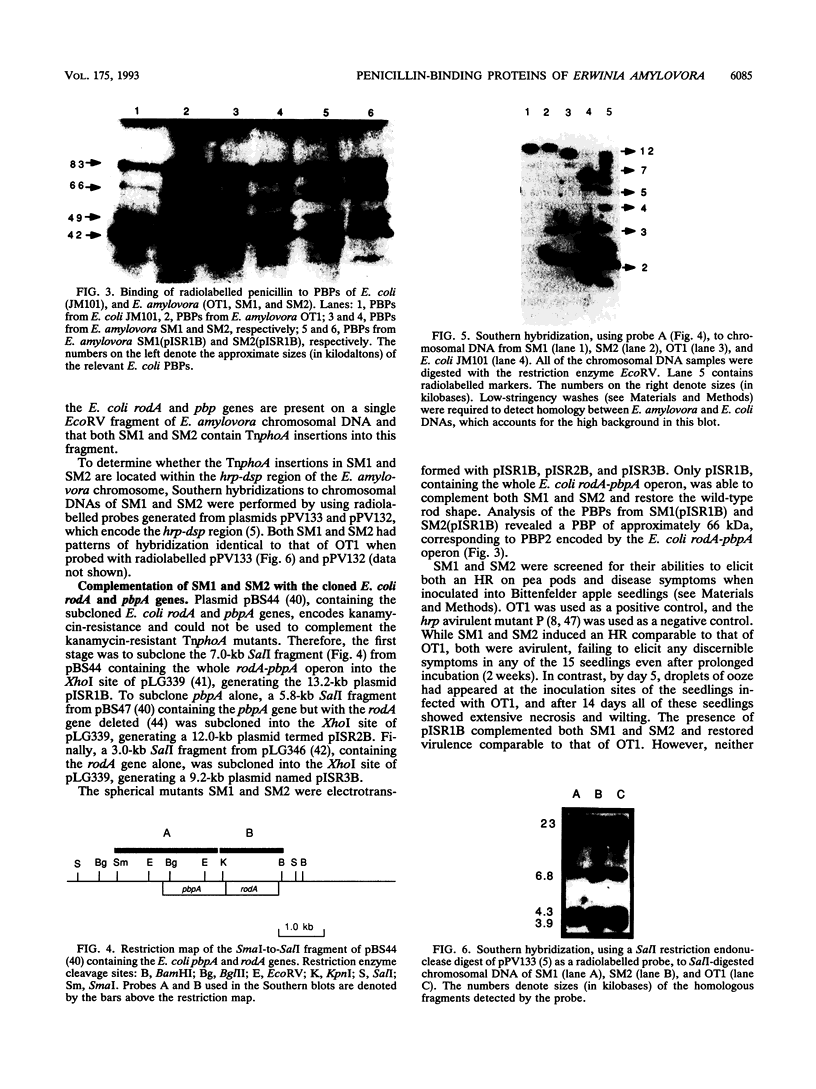
Radiolabelled penicillin G was used to examine penicillin-binding proteins (PBPs) from Erwinia amylovora (OT1). This procedure identified seven PBPs with molecular masses ranging from 22 to 83 kDa. E. amylovora PBPs were compared with those from Escherichia coli (JM101) and from two spherical, avirulent TnphoA mutants derived from OT1. Radiolabelled penicillin G bound to only six proteins from the spherical mutants which lacked a 69-kDa PBP. The spherical mutants could be complemented by the cloned E. coli pbpA-rodA operon, which restored both cell shape and virulence to apple seedlings. This suggested that the E. amylovora 69-kDa PBP is probably the functional equivalent of the E. coli PBP2 protein. Southern blot analysis using the E. coli rodA and pbpA genes as radiolabelled probes showed that TnphoA had inserted into the E. amylovora equivalent of the E. coli rodA-pbpA operon. Southern blots to chromosomal DNAs of the two spherical mutants, using the cloned hrp and dsp genes from E. amylovora as radiolabelled probes, confirmed that the TnphoA insertions were not located in the region of the E. amylovora chromosome postulated to encode known virulence factors. Both of the spherical TnphoA mutants synthesized amounts of extracellular polysaccharide equivalent to those synthesized by the wild-type strain (OT1), were resistant to lysis in distilled water and to lysozyme, and elicited the hypersensitive response on nonhost plants. These results indicate a possible role for cell shape in the virulence of this plant pathogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Ohta T., Matsuzawa H. A water-soluble form of penicillin-binding protein 2 of Escherichia coli constructed by site-directed mutagenesis. FEBS Lett. 1987 Dec 21;226(1):150–154. doi: 10.1016/0014-5793(87)80569-0. [DOI] [PubMed] [Google Scholar]
- Asoh S., Matsuzawa H., Ishino F., Strominger J. L., Matsuhashi M., Ohta T. Nucleotide sequence of the pbpA gene and characteristics of the deduced amino acid sequence of penicillin-binding protein 2 of Escherichia coli K12. Eur J Biochem. 1986 Oct 15;160(2):231–238. doi: 10.1111/j.1432-1033.1986.tb09961.x. [DOI] [PubMed] [Google Scholar]
- Asoh S., Matsuzawa H., Matsuhashi M., Ohta T. Molecular cloning and characterization of the genes (pbpA and rodA) responsible for the rod shape of Escherichia coli K-12: analysis of gene expression with transposon Tn5 mutagenesis and protein synthesis directed by constructed plasmids. J Bacteriol. 1983 Apr;154(1):10–16. doi: 10.1128/jb.154.1.10-16.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barny M. A., Guinebretière M. H., Marçais B., Coissac E., Paulin J. P., Laurent J. Cloning of a large gene cluster involved in Erwinia amylovora CFBP1430 virulence. Mol Microbiol. 1990 May;4(5):777–786. doi: 10.1111/j.1365-2958.1990.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Park W., Tomioka S., Tamaki S., Takase I., Kunugita K., Matsuzawa H., Asoh S., Ohta T., Spratt B. G. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986 May 25;261(15):7024–7031. [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Asoh S., Kunai K., Muraiso K., Takasuga A., Ohta T. Nucleotide sequence of the rodA gene, responsible for the rod shape of Escherichia coli: rodA and the pbpA gene, encoding penicillin-binding protein 2, constitute the rodA operon. J Bacteriol. 1989 Jan;171(1):558–560. doi: 10.1128/jb.171.1.558-560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Bouloc P., Niki H., D'Ari R., Hiraga S., Jaffé A. Penicillin-binding protein 2 is essential in wild-type Escherichia coli but not in lov or cya mutants. J Bacteriol. 1989 Jun;171(6):3025–3030. doi: 10.1128/jb.171.6.3025-3030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Mahadevan S. Penicillin-binding proteins of bdellovibrios. J Bacteriol. 1988 Aug;170(8):3750–3751. doi: 10.1128/jb.170.8.3750-3751.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W. J., Saz A. K. Differential binding of penicillin by membrane fractions from penicillin-susceptible and -resistant gonococci. Antimicrob Agents Chemother. 1978 Apr;13(4):589–597. doi: 10.1128/aac.13.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Broome-Smith J. K., Edelman A., Spratt B. G. Organization and subcloning of the dacA-rodA-pbpA cluster of cell shape genes in Escherichia coli. J Bacteriol. 1983 Aug;155(2):847–853. doi: 10.1128/jb.155.2.847-853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Pratt J. M., Spratt B. G. Identification of the rodA gene product of Escherichia coli. J Bacteriol. 1983 Aug;155(2):854–859. doi: 10.1128/jb.155.2.854-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuzawa H., Matsuhashi M. Cluster of mrdA and mrdB genes responsible for the rod shape and mecillinam sensitivity of Escherichia coli. J Bacteriol. 1980 Jan;141(1):52–57. doi: 10.1128/jb.141.1.52-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M., Doi M., Tamaki S., Park W., Nakajima-Iijima S., Matsuhashi M. Mutant isolation and molecular cloning of mre genes, which determine cell shape, sensitivity to mecillinam, and amount of penicillin-binding proteins in Escherichia coli. J Bacteriol. 1987 Nov;169(11):4935–4940. doi: 10.1128/jb.169.11.4935-4940.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A., Rigg G., Hyman S. C., James R., Roberts I. S. Generation and characterization of a monoclonal antibody specific for the major thiol-activated cysteine proteinase of Porphyromonas gingivalis W83. Lett Appl Microbiol. 1992 Nov;15(5):202–206. doi: 10.1111/j.1472-765x.1992.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Wei Z. M., Laby R. J., Zumoff C. H., Bauer D. W., He S. Y., Collmer A., Beer S. V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992 Jul 3;257(5066):85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]