Abstract
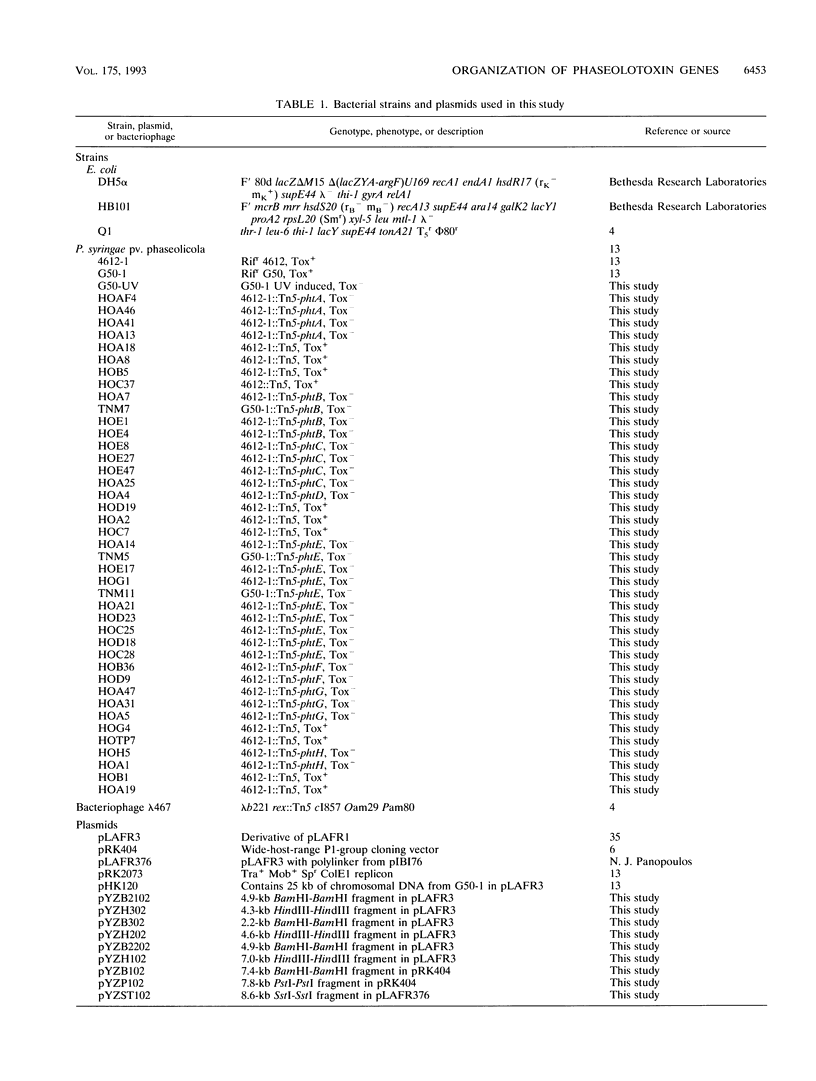
Phaseolotoxin [N delta(N'-sulfo-diaminophosphinyl)-ornithyl-alanyl- homoarginine] produced by Pseudomonas syringae pv. phaseolicola, the bean halo blight pathogen, is a potent inhibitor of ornithine carbamoyltransferase (OCT). Inhibition of OCT in infected plants leads to chlorosis and growth inhibition. A genomic cosmid clone, pHK120, containing a 25-kb fragment of DNA from a wild-type strain of P. syringae pv. phaseolicola restores toxin production in Tox- mutants. Tn5 mutagenesis of pHK120 and marker exchange of pHK120::Tn5 plasmids in the wild-type strain resulted in the isolation of 39 chromosomal mutants that harbor Tn5 insertions at known positions. Toxin bioassays revealed that 28 of the mutants, with Tn5 insertions distributed throughout the insert of pHK120, were Tox-, indicating that a functional locus for toxin production in each mutant was inactivated. Complementation analysis was done by testing for toxin production strains that carried a genomic Tn5 at one location and a plasmid-borne Tn5 at another location (pair complementation). Pair complementation analysis of nine marker exchange mutants and a random genomic Tn5 mutant revealed that there are a minimum of eight toxin loci (phtA through phtH) in pHK120. Mutants carrying Tn5 insertions in the phtA, phtD, and phtF loci were complemented by deletion subclones containing fragments from pHK120; mutants carrying Tn5 insertions in the phtC locus were partially complemented by a subclone, and mutants carrying Tn5 insertions in the phtB, phtE, phtG, and phtH loci were not complemented by any of the available subclones. A comparison of the insert from pHK120 with that from pRCP17, a clone reported previously (R. C. Peet, P. B. Lindgren, D. K. Wills, and N. J. Panopoulos, J. Bacteriol. 166:1096-1105, 1986) by another laboratory to contain some of the phaseolotoxin genes and the phaseolotoxin-resistant OCT gene, revealed that the inserts in these two cosmids overlap but differ in important respects.
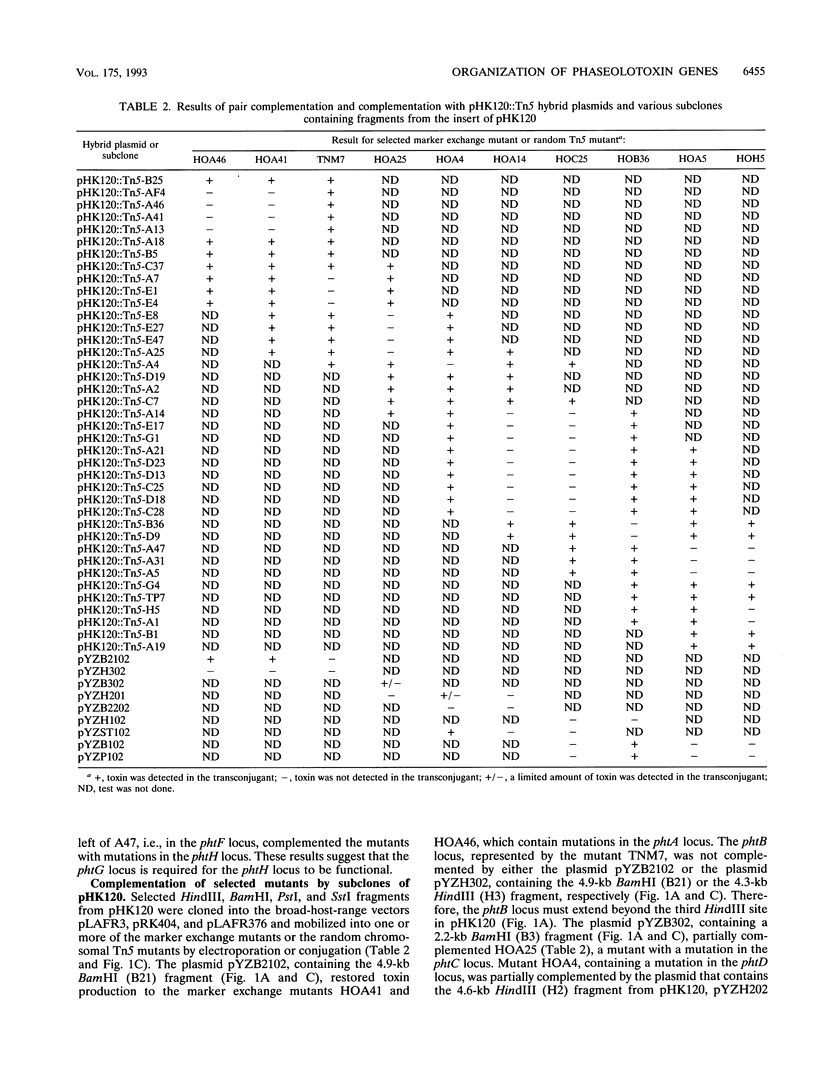
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzai H., Murakami T., Imai S., Satoh A., Nagaoka K., Thompson C. J. Transcriptional regulation of bialaphos biosynthesis in Streptomyces hygroscopicus. J Bacteriol. 1987 Aug;169(8):3482–3488. doi: 10.1128/jb.169.8.3482-3488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Gutterson N. I., Layton T. J., Ziegle J. S., Warren G. J. Molecular cloning of genetic determinants for inhibition of fungal growth by a fluorescent pseudomonad. J Bacteriol. 1986 Mar;165(3):696–703. doi: 10.1128/jb.165.3.696-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziloukas E., Panopoulos N. J. Origin, structure, and regulation of argK, encoding the phaseolotoxin-resistant ornithine carbamoyltransferase in Pseudomonas syringae pv. phaseolicola, and functional expression of argK in transgenic tobacco. J Bacteriol. 1992 Sep;174(18):5895–5909. doi: 10.1128/jb.174.18.5895-5909.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs T. W., Egelhoff T. T., Long S. R. Physical and genetic map of a Rhizobium meliloti nodulation gene region and nucleotide sequence of nodC. J Bacteriol. 1985 May;162(2):469–476. doi: 10.1128/jb.162.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kamdar H. V., Rowley K. B., Clements D., Patil S. S. Pseudomonas syringae pv. phaseolicola genomic clones harboring heterologous DNA sequences suppress the same phaseolotoxin-deficient mutants. J Bacteriol. 1991 Feb;173(3):1073–1079. doi: 10.1128/jb.173.3.1073-1079.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinscherf T. G., Coleman R. H., Barta T. M., Willis D. K. Cloning and expression of the tabtoxin biosynthetic region from Pseudomonas syringae. J Bacteriol. 1991 Jul;173(13):4124–4132. doi: 10.1128/jb.173.13.4124-4132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- Mitchell R. E., Bieleski R. L. Involvement of phaseolotoxin in halo blight of beans: transport and conversion to functional toxin. Plant Physiol. 1977 Nov;60(5):723–729. doi: 10.1104/pp.60.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. K., Chatterjee A. K. Genetic organization and regulation of proteins associated with production of syringotoxin by Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5689–5697. doi: 10.1128/jb.170.12.5689-5697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. K., Chatterjee A. K. Isolation and characterization of Tn5 insertion mutants of Pseudomonas syringae pv. syringae altered in the production of the peptide phytotoxin syringotoxin. J Bacteriol. 1985 Oct;164(1):14–18. doi: 10.1128/jb.164.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueda G., Van den Broeck G., Saucedo O., Bailey A. M., Alvarez-Morales A., Herrera-Estrella L. Isolation and characterization of the gene from Pseudomonas syringae pv. phaseolicola encoding the phaseolotoxin-insensitive ornithine carbamoyltransferase. Mol Gen Genet. 1990 Jul;222(2-3):461–466. doi: 10.1007/BF00633857. [DOI] [PubMed] [Google Scholar]
- Patil S. S. Inhibition of Ornithin Carbamyl Transferase from Bean Plants by the Toxin of Pseudomonas phaseolicola. Plant Physiol. 1970 Nov;46(5):752–753. doi: 10.1104/pp.46.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet R. C., Lindgren P. B., Willis D. K., Panopoulos N. J. Identification and cloning of genes involved in phaseolotoxin production by Pseudomonas syringae pv. "phaseolicola". J Bacteriol. 1986 Jun;166(3):1096–1105. doi: 10.1128/jb.166.3.1096-1105.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet R. C., Panopoulos N. J. Ornithine carbamoyltransferase genes and phaseolotoxin immunity in Pseudomonas syringae pv. phaseolicola. EMBO J. 1987 Dec 1;6(12):3585–3591. doi: 10.1002/j.1460-2075.1987.tb02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley K. B., Clements D. E., Mandel M., Humphreys T., Patil S. S. Multiple copies of a DNA sequence from Pseudomonas syringae pathovar phaseolicola abolish thermoregulation of phaseolotoxin production. Mol Microbiol. 1993 May;8(3):625–635. doi: 10.1111/j.1365-2958.1993.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991 Jul;173(13):4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. W., Gross D. C. Physical and functional analyses of the syrA and syrB genes involved in syringomycin production by Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5680–5688. doi: 10.1128/jb.170.12.5680-5688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Park S. K., Rodgers C., Mitchell R. E., Bender C. L. Physical and functional characterization of the gene cluster encoding the polyketide phytotoxin coronatine in Pseudomonas syringae pv. glycinea. J Bacteriol. 1992 Mar;174(6):1837–1843. doi: 10.1128/jb.174.6.1837-1843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]


