Abstract
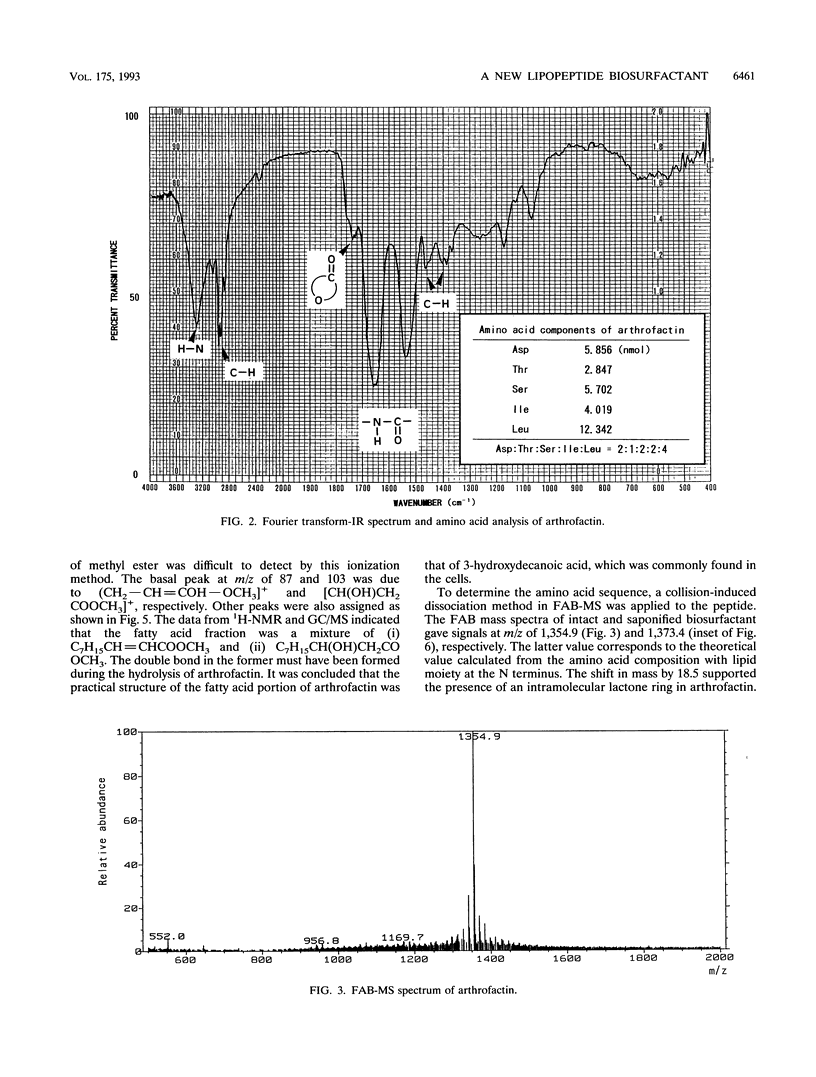
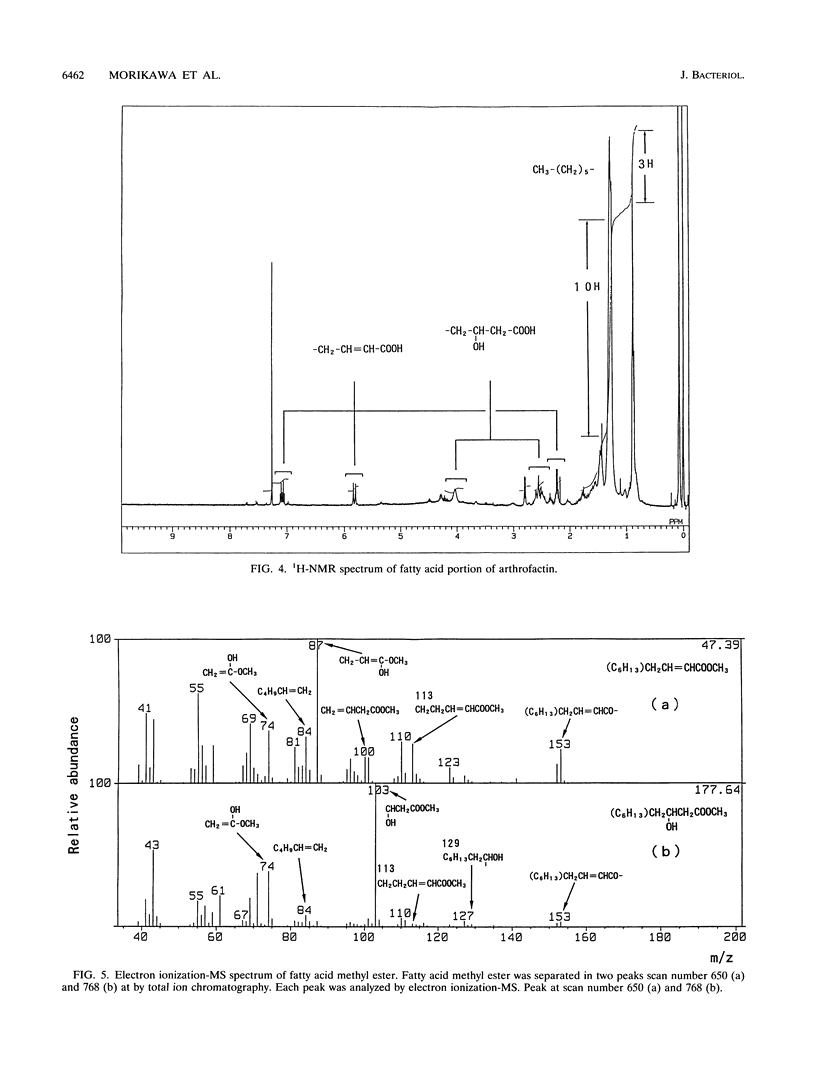
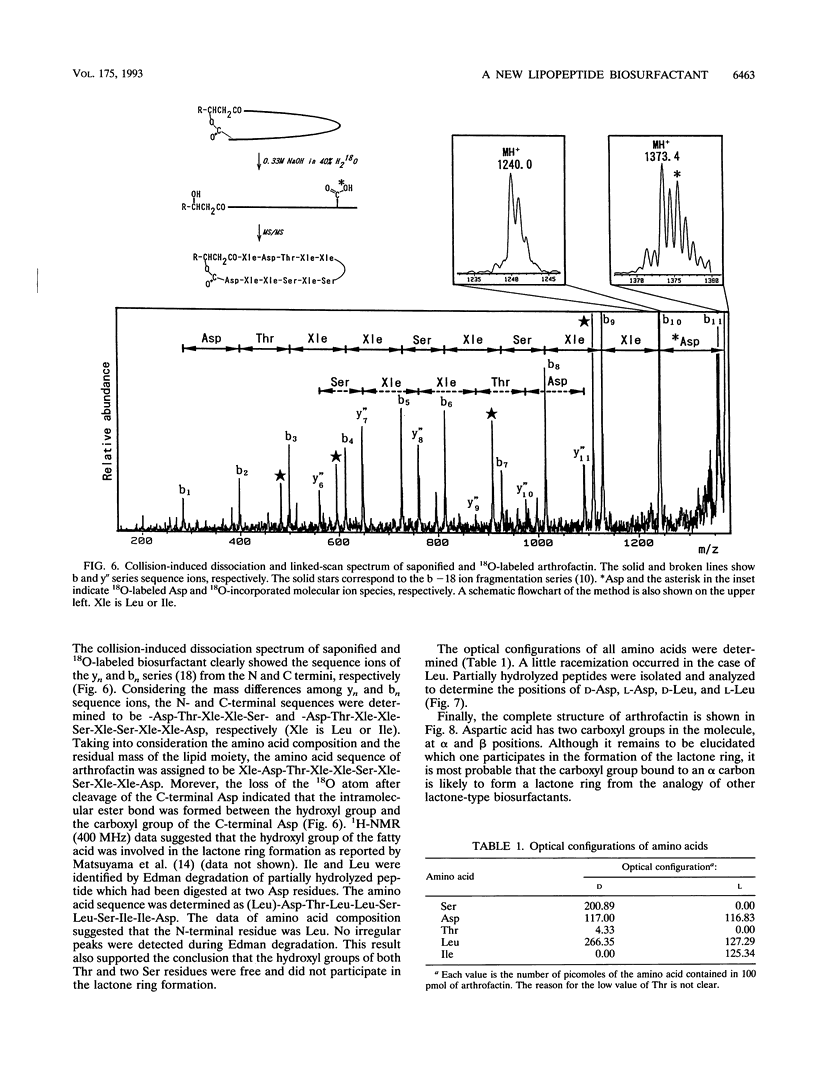
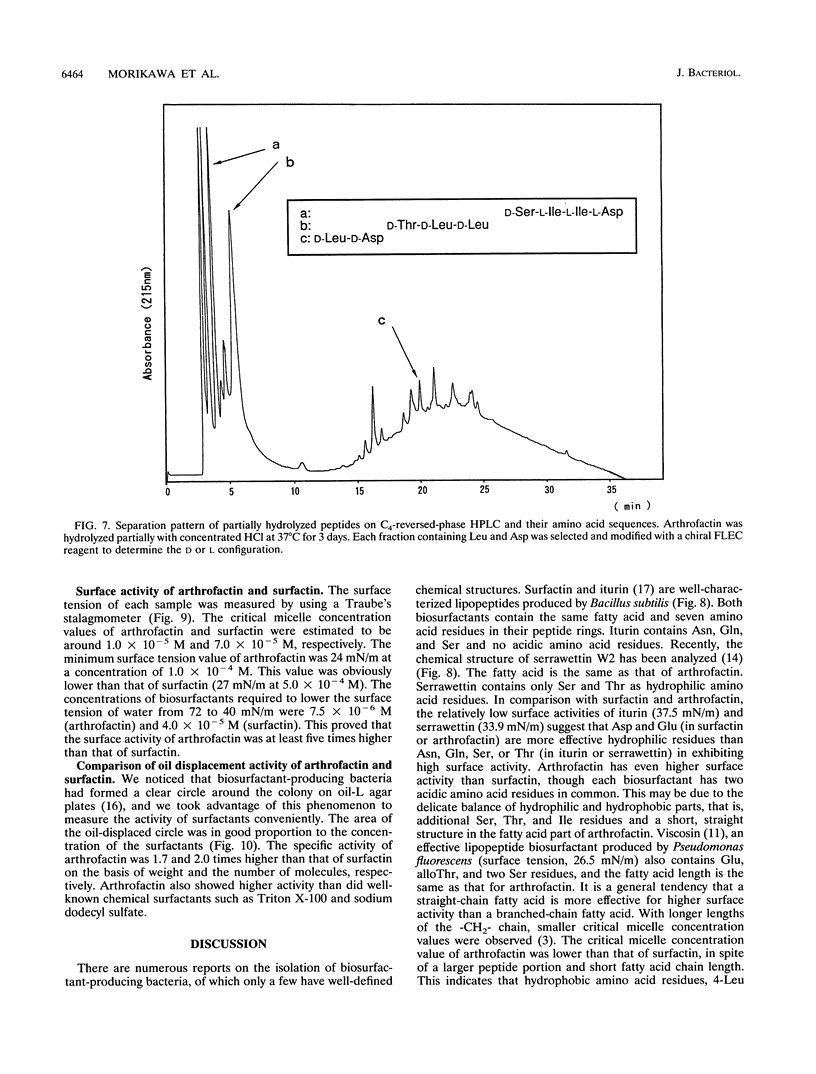
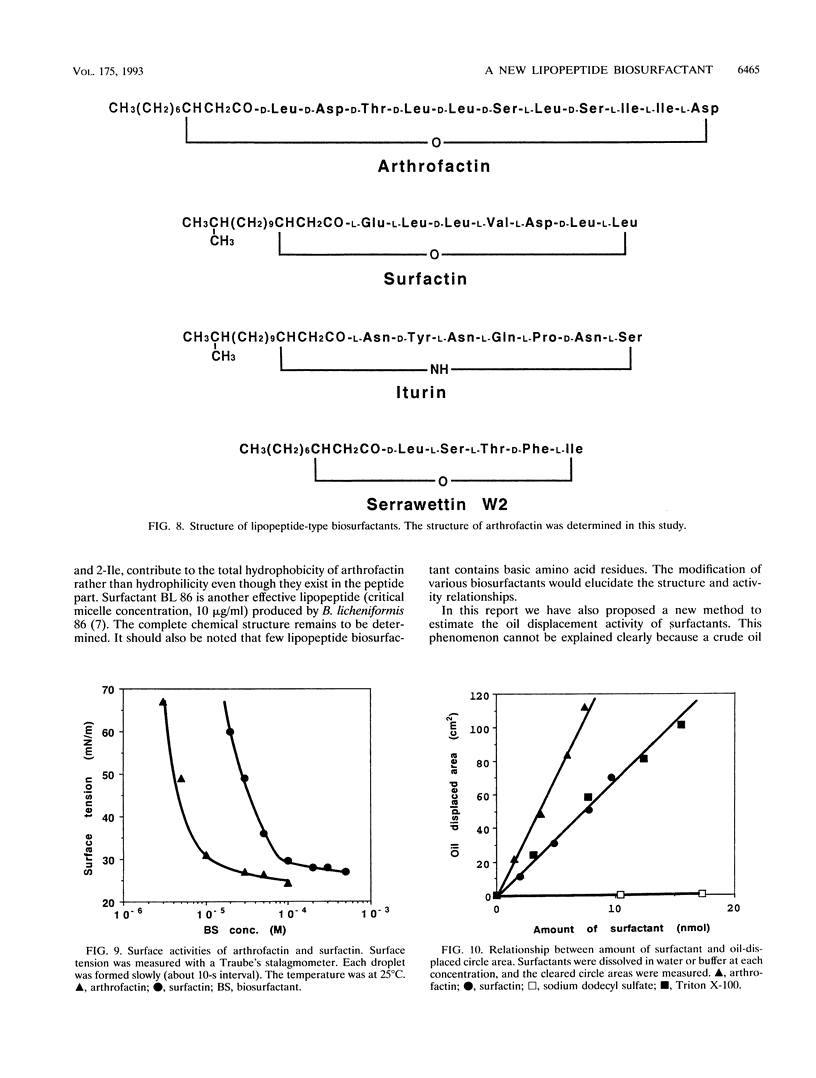
A biosurfactant termed arthrofactin produced by Arthrobacter species strain MIS38 was purified and chemically characterized as 3-hydroxydecanoyl-D-leucyl-D-asparagyl-D-threonyl-D- leucyl-D-leucyl-D-seryl-L-leucyl-D-seryl-L-isoleucyl-L-isoleucyl-L-as paragyl lactone. Surface activity of arthrofactin was examined, with surfactin as a control. Critical micelle concentration values of arthrofactin and surfactin were around 1.0 x 10(-5) M and 7.0 x 10(-5) M at 25 degrees C, respectively. Arthrofactin was found to be five to seven times more effective than surfactin. The minimum surface tension value of arthrofactin was 24 mN/m at a concentration higher than the critical micelle concentration. According to the oil displacement assay, arthrofactin was a better oil remover than synthetic surfactants, such as Triton X-100 and sodium dodecyl sulfate. Arthrofactin is one of the most effective lipopeptide biosurfactants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Kakinuma A., Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun. 1968 May 10;31(3):488–494. doi: 10.1016/0006-291x(68)90503-2. [DOI] [PubMed] [Google Scholar]
- Einarsson S., Josefsson B., Möller P., Sanchez D. Separation of amino acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography. Anal Chem. 1987 Apr 15;59(8):1191–1195. doi: 10.1021/ac00135a025. [DOI] [PubMed] [Google Scholar]
- Fiechter A. Biosurfactants: moving towards industrial application. Trends Biotechnol. 1992 Jun;10(6):208–217. doi: 10.1016/0167-7799(92)90215-h. [DOI] [PubMed] [Google Scholar]
- Gutierrez J. R., Erickson L. E. Hydrocarbon uptake in hydrocarbon fermentations. Biotechnol Bioeng. 1977 Sep;19(9):1331–1349. doi: 10.1002/bit.260190907. [DOI] [PubMed] [Google Scholar]
- Hug H., Blanch H. W., Fiechter A. The functional role of lipids in hydrocarbon assimilation. Biotechnol Bioeng. 1974 Jul;16(7):965–985. doi: 10.1002/bit.260160709. [DOI] [PubMed] [Google Scholar]
- Jenny K., Käppeli O., Fiechter A. Biosurfactants from Bacillus licheniformis: structural analysis and characterization. Appl Microbiol Biotechnol. 1991 Oct;36(1):5–13. doi: 10.1007/BF00164690. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Martin S. A., Biemann K., Stults J. T., Watson J. T. Novel fragmentation process of peptides by collision-induced decomposition in a tandem mass spectrometer: differentiation of leucine and isoleucine. Anal Chem. 1987 Nov 1;59(21):2621–2625. doi: 10.1021/ac00148a019. [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Lang S., Wagner F., Witte L., Wray V. Formation and identification of interfacial-active glycolipids from resting microbial cells. Appl Environ Microbiol. 1984 Sep;48(3):610–617. doi: 10.1128/aem.48.3.610-617.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Kaneda K., Nakagawa Y., Isa K., Hara-Hotta H., Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992 Mar;174(6):1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Javaheri M., Nagle D. P., Jr Properties of the biosurfactant produced by Bacillus licheniformis strain JF-2. J Ind Microbiol. 1990 Apr-May;5(2-3):95–101. doi: 10.1007/BF01573858. [DOI] [PubMed] [Google Scholar]
- Peypoux F., Guinand M., Michel G., Delcambe L., Das B. C., Lederer E. Structure of iturine A, a peptidolipid antibiotic from Bacillus subtilis. Biochemistry. 1978 Sep 19;17(19):3992–3996. doi: 10.1021/bi00612a018. [DOI] [PubMed] [Google Scholar]
- Roepstorff P., Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984 Nov;11(11):601–601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Takao T., Hori H., Okamoto K., Harada A., Kamachi M., Shimonishi Y. Facile assignment of sequence ions of a peptide labelled with 18O at the carboxyl terminus. Rapid Commun Mass Spectrom. 1991 Jul;5(7):312–315. doi: 10.1002/rcm.1290050703. [DOI] [PubMed] [Google Scholar]



