Abstract
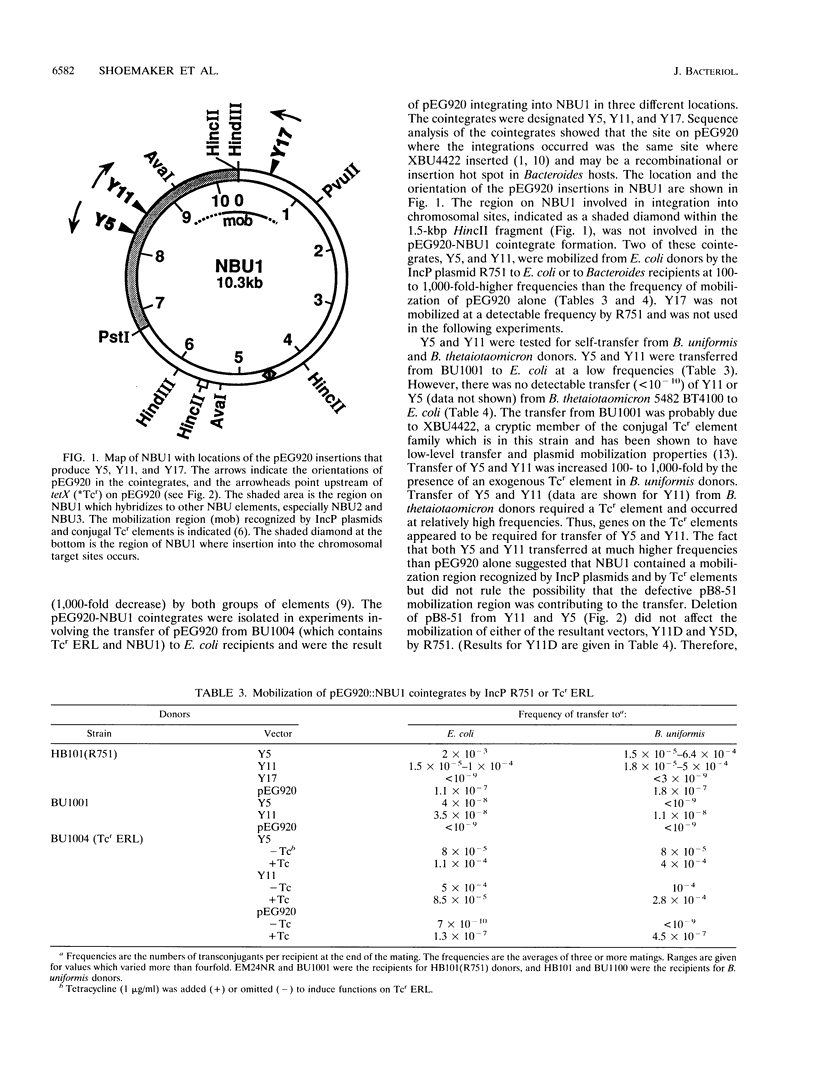
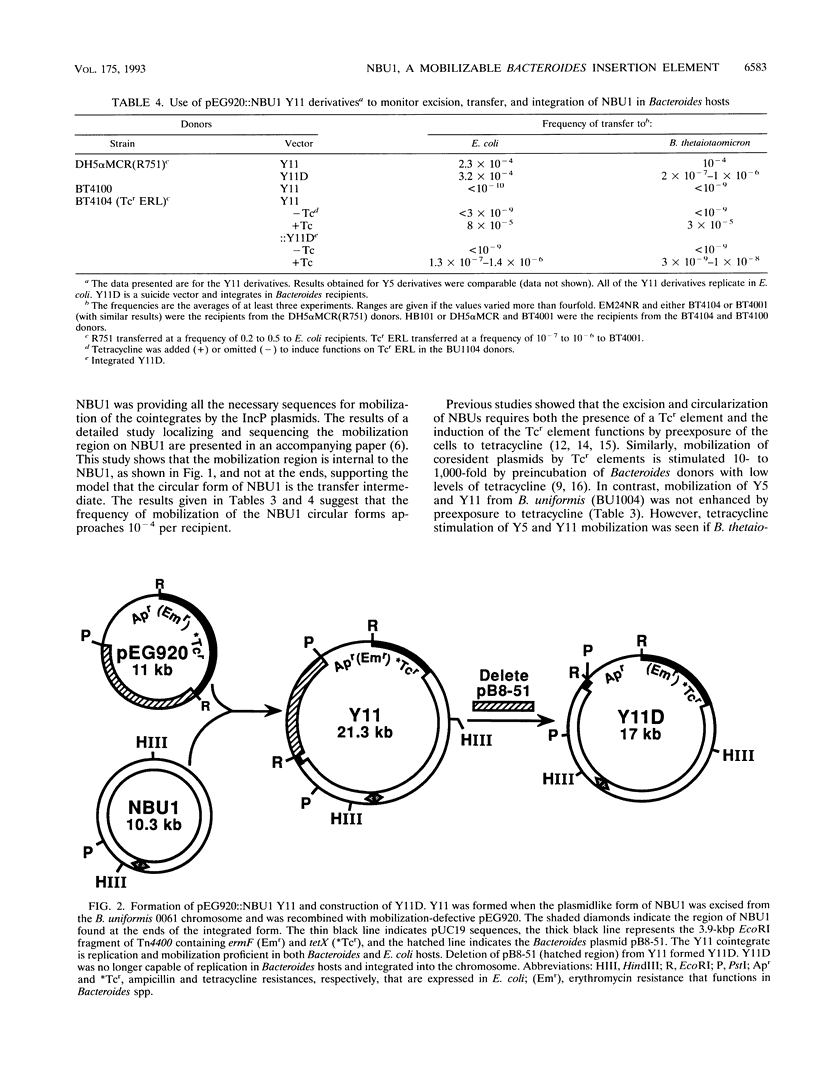
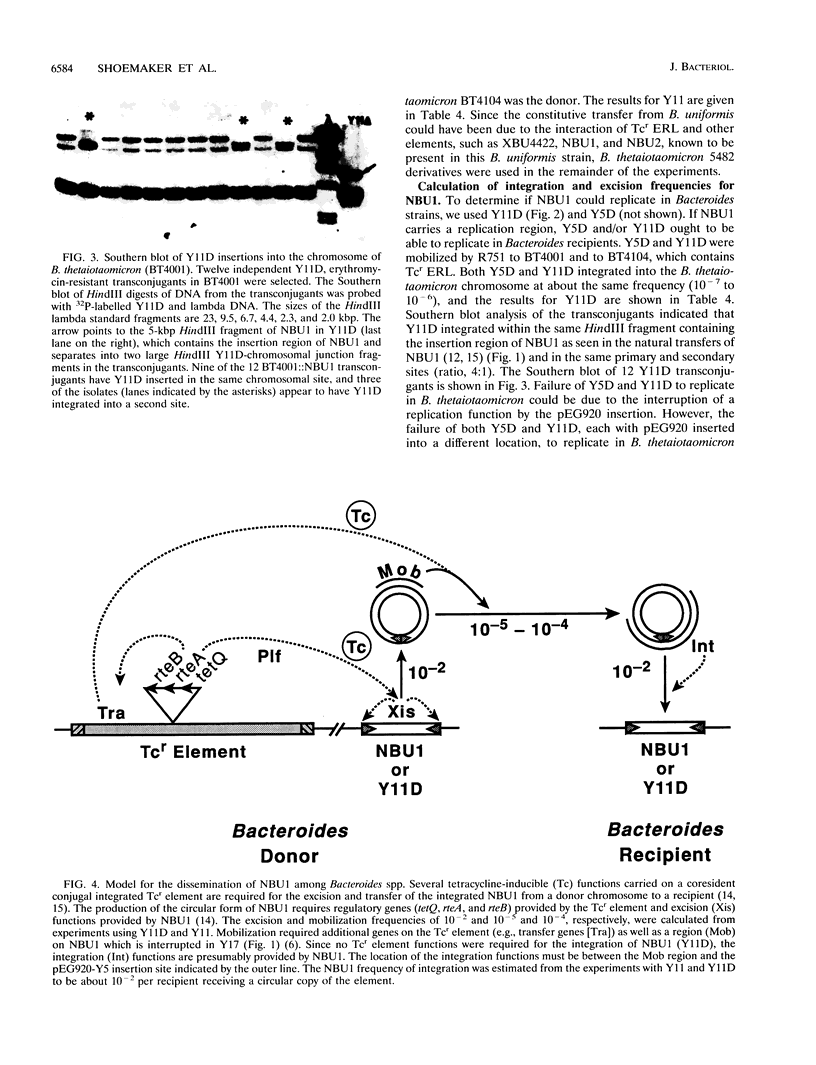
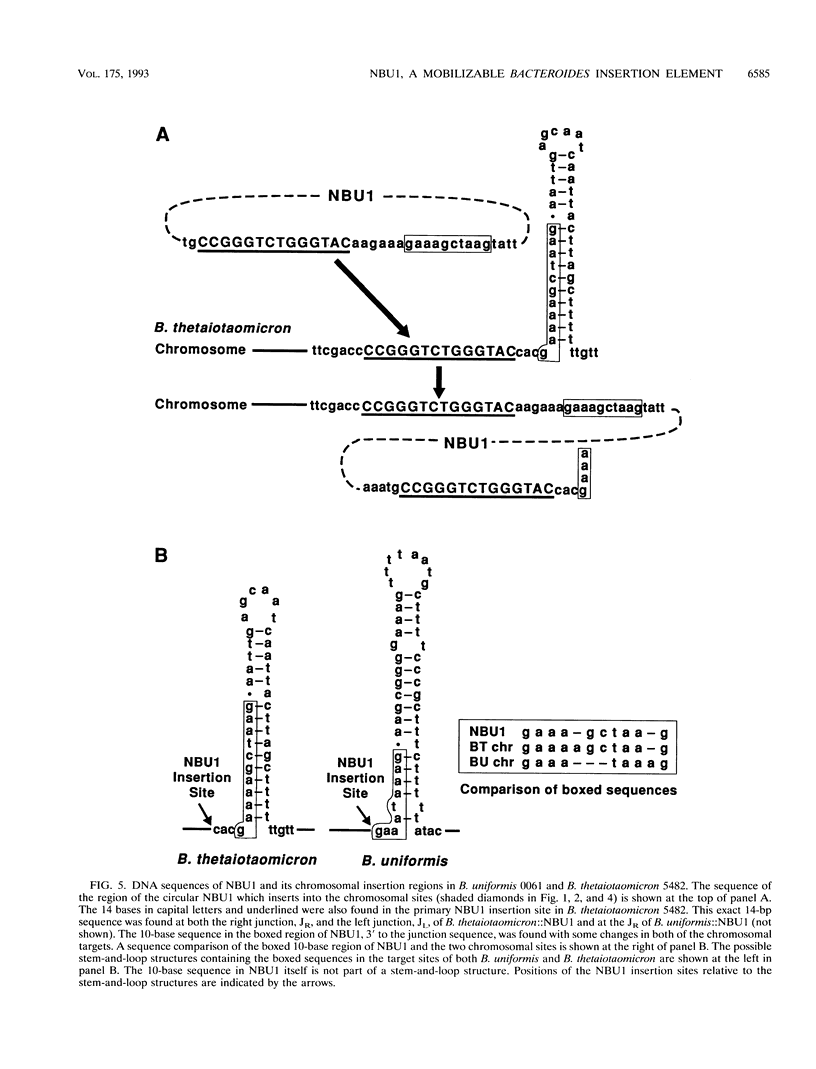
The Bacteroides species harbor a family of conjugative transposons called tetracycline resistance elements (Tcr elements) that transfer themselves from the chromosome of a donor to the chromosome of a recipient, mobilize coresident plasmids, and also mediate the excision and circularization of members of a family of 10- to 12-kbp insertion elements which share a small region of DNA homology and are called NBUs (for nonreplicating Bacteroides units). The NBUs are sometimes cotransferred with Tcr elements, and it was postulated previously that the excised circular forms of the NBUs were plasmidlike forms and were transferred like plasmids and then integrated into the recipient chromosome. We used chimeric plasmids containing one of the NBUs, NBU1, and a Bacteroides-Escherichia coli shuttle vector to show that this hypothesis is probably correct. NBU1 contained a region that allowed mobilization by both the Tcr elements and IncP plasmids, and we used these conjugal elements to allow us to estimate the frequencies of excision, mobilization, and integration of NBU1 in Bacteroides hosts to be approximately 10(-2), 10(-5) to 10(-4), and 10(-2), respectively. Although functions on the Tcr elements were required for the excision-circularization and mobilization of NBU1, no Tcr element functions were required for integration into the recipient chromosome. Analysis of the DNA sequences at the integration region of the circular form of NBU1, the primary insertion site in the Bacteroides thetaiotaomicron 5482 chromosome, and the resultant NBU1-chromosome junctions showed that NBU1 appeared to integrate into the primary insertion site by recombining within an identical 14-bp sequence present on both NBU1 and the target, thus leaving a copy of the 14-bp sequence at both junctions. The apparent integration mechanism and the target selection of NBU1 were different from those of both XBU4422, the only member of the conjugal Tcr elements for which these sequences are known, and Tn4399, a mobilizable Bacteroides transposon. The NBUs appear to be a distinct type of mobilizable insertion element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedzyk L. A., Shoemaker N. B., Young K. E., Salyers A. A. Insertion and excision of Bacteroides conjugative chromosomal elements. J Bacteriol. 1992 Jan;174(1):166–172. doi: 10.1128/jb.174.1.166-172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Hecht D. W., Malamy M. H. Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. J Bacteriol. 1989 Jul;171(7):3603–3608. doi: 10.1128/jb.171.7.3603-3608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D. W., Thompson J. S., Malamy M. H. Characterization of the termini and transposition products of Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5340–5344. doi: 10.1073/pnas.86.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. Y., Shoemaker N. B., Salyers A. A. Characterization of the mobilization region of a Bacteroides insertion element (NBU1) that is excised and transferred by Bacteroides conjugative transposons. J Bacteriol. 1993 Oct;175(20):6588–6598. doi: 10.1128/jb.175.20.6588-6598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. A cryptic 65-kilobase-pair transposonlike element isolated from Bacteroides uniformis has homology with Bacteroides conjugal tetracycline resistance elements. J Bacteriol. 1990 Apr;172(4):1694–1702. doi: 10.1128/jb.172.4.1694-1702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Facilitated transfer of IncP beta R751 derivatives from the chromosome of Bacteroides uniformis to Escherichia coli recipients by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1987 Jul;169(7):3160–3167. doi: 10.1128/jb.169.7.3160-3167.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Tetracycline-dependent appearance of plasmidlike forms in Bacteroides uniformis 0061 mediated by conjugal Bacteroides tetracycline resistance elements. J Bacteriol. 1988 Apr;170(4):1651–1657. doi: 10.1128/jb.170.4.1651-1657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Sanders J. M., Shoemaker N. B., Salyers A. A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992 May;174(9):2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Shoemaker N. B., Salyers A. A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990 Aug;172(8):4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine P. J., Shoemaker N. B., Salyers A. A. Mobilization of Bacteroides plasmids by Bacteroides conjugal elements. J Bacteriol. 1988 Mar;170(3):1319–1324. doi: 10.1128/jb.170.3.1319-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]