Abstract
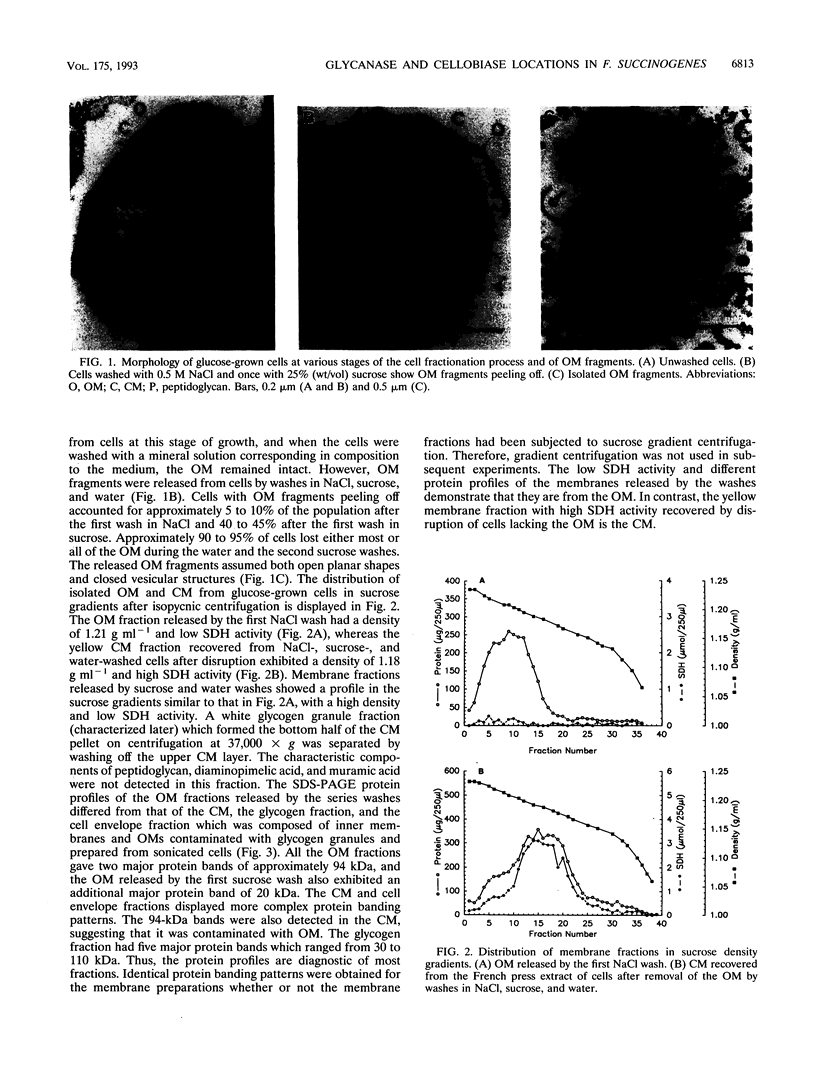
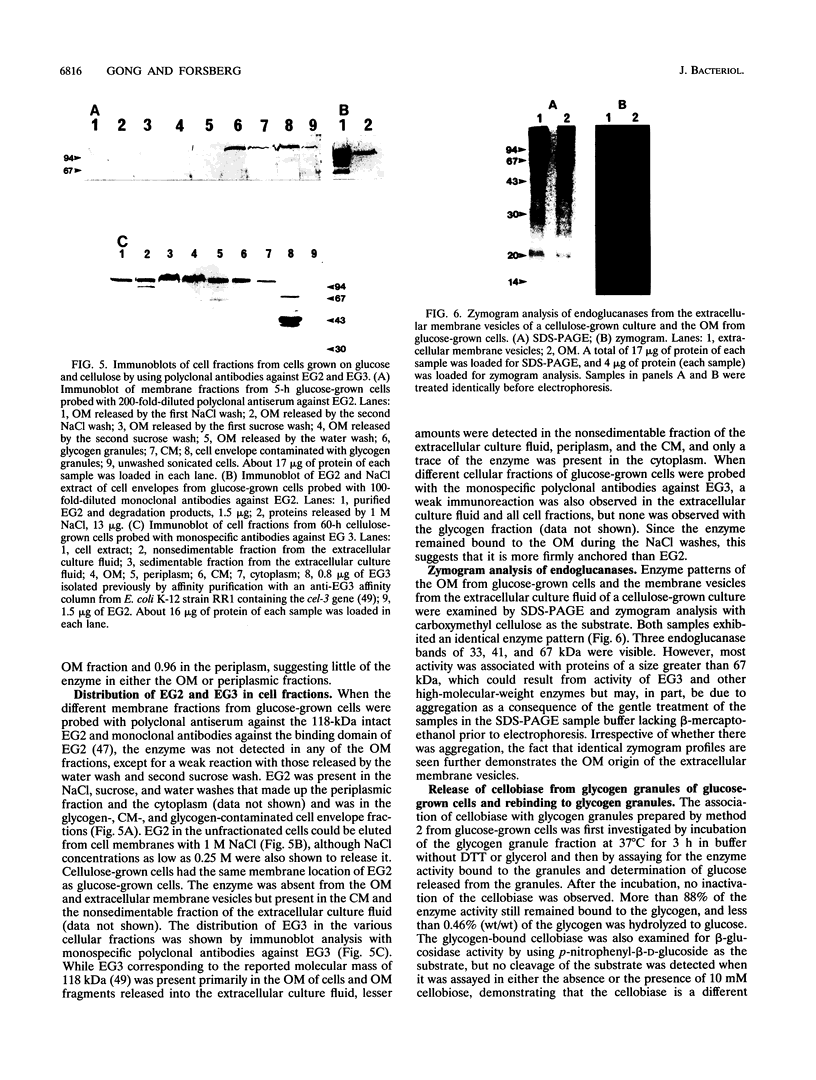
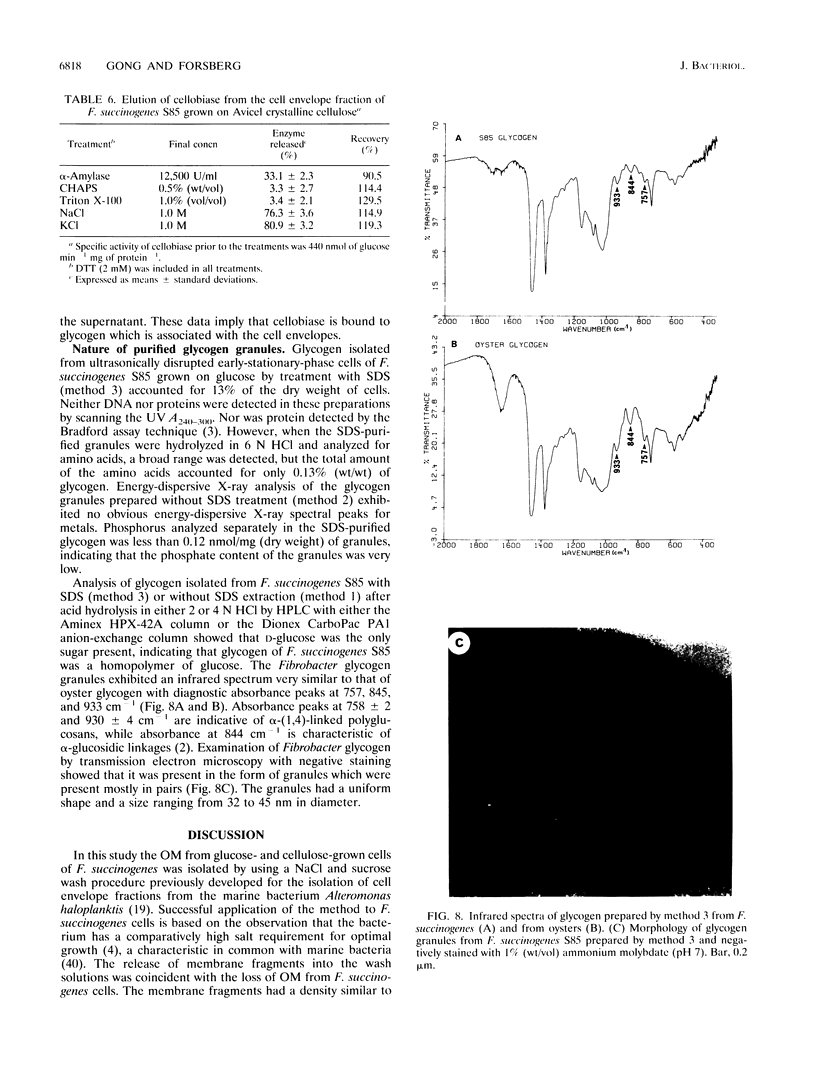
The outer membrane (OM) of Fibrobacter succinogenes was isolated by a combination of salt, sucrose, and water washes from whole cells grown on either glucose or cellulose. The cytoplasmic membrane (CM) was isolated from OM-depleted cells after disruption with a French press. The OM and membrane vesicles isolated from the extracellular culture fluid of cellulose-grown cells had a higher density, much lower succinate dehydrogenase activity, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiles different from those of the CM. The OM from both glucose- and cellulose-grown cells and the extracellular membrane vesicles from cellulose-grown cultures exhibited higher endoglucanase, xylanase, and acetylesterase activities than the CM and other cell fractions. Endoglucanase 2 was absent from the isolated OM fractions of glucose- and cellulose-grown cells and from the extracellular membrane vesicles of cellulose-grown cells but was present in the CM and intracellular glycogen granule fractions, while endoglucanase 3 was enriched in the OM. Cellobiosidase was located primarily in the periplasm as previously reported, while cellobiase was mainly present in the glycogen granule fraction of glucose-grown cells and in a nongranular glycogen and CM complex in cellulose-grown cells. The cellobiase was not eluted from glycogen granules by cellobiose, maltose, and maltotriose nor from either the granules or the cell membranes by nondenaturing detergents but was eluted from both glycogen granules and cell membranes by high concentrations of salts. The eluted cellobiase rebound almost quantitatively when diluted and mixed with purified glycogen granules but exhibited a low affinity for Avicel cellulose. Thus, we have documented a method for isolation of OM from F. succinogenes, identified the OM origin of the extracellular membrane vesicles, and located glycanases and cellobiase in membrane and glycogen fractions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BARKER S. A., BOURNE E. J., WHIFFEN D. H. Use of infrared analysis in the determination of carbohydrate structure. Methods Biochem Anal. 1956;3:213–245. doi: 10.1002/9780470110195.ch7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan C. J., Mitchell W. J. Two beta-glucosidase activities in Fibrobacter succinogenes S85. J Appl Bacteriol. 1992 Sep;73(3):243–250. doi: 10.1111/j.1365-2672.1992.tb02984.x. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Roberts D. W., Costerton J. W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can J Microbiol. 1973 Dec;19(12):1501–1506. doi: 10.1139/m73-244. [DOI] [PubMed] [Google Scholar]
- Clarke A. J. Chemical modification of a beta-glucosidase from Schizophyllum commune: evidence for essential carboxyl groups. Biochim Biophys Acta. 1990 Sep 3;1040(2):145–152. doi: 10.1016/0167-4838(90)90069-r. [DOI] [PubMed] [Google Scholar]
- Clarke A. J., Sarabia V., Keenleyside W., MacLachlan P. R., Whitfield C. The compositional analysis of bacterial extracellular polysaccharides by high-performance anion-exchange chromatography. Anal Biochem. 1991 Nov 15;199(1):68–74. doi: 10.1016/0003-2697(91)90270-4. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Groleau D. Stability of the endo-beta-1,4-glucanase and beta-1,4-glucosidase from Bacteroides succinogenes. Can J Microbiol. 1982 Jan;28(1):144–148. doi: 10.1139/m82-017. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Lam K. Use of adenosine 5'-triphosphate as an indicator of the microbiota biomass in rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):528–537. doi: 10.1128/aem.33.3.528-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet G., Forano E., Dauphin G., Delort A. M. Futile cycling of glycogen in Fibrobacter succinogenes as shown by in situ 1H-NMR and 13C-NMR investigation. Eur J Biochem. 1992 Jul 1;207(1):155–162. doi: 10.1111/j.1432-1033.1992.tb17032.x. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Gräbnitz F., Seiss M., Rücknagel K. P., Staudenbauer W. L. Structure of the beta-glucosidase gene bglA of Clostridium thermocellum. Sequence analysis reveals a superfamily of cellulases and beta-glycosidases including human lactase/phlorizin hydrolase. Eur J Biochem. 1991 Sep 1;200(2):301–309. doi: 10.1111/j.1432-1033.1991.tb16186.x. [DOI] [PubMed] [Google Scholar]
- Harvey M., Forsberg C. W., Beveridge T. J., Pos J., Ogilvie J. R. Methanogenic activity and structural characteristics of the microbial biofilm on a needle-punched polyester support. Appl Environ Microbiol. 1984 Sep;48(3):633–638. doi: 10.1128/aem.48.3.633-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Cellulose digestion and cellulase regulation and distribution in Fibrobacter succinogenes subsp. succinogenes S85. Appl Environ Microbiol. 1990 May;56(5):1221–1228. doi: 10.1128/aem.56.5.1221-1228.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Purification and Comparison of the Periplasmic and Extracellular Forms of the Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1988 Jun;54(6):1488–1493. doi: 10.1128/aem.54.6.1488-1493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W., Thomas D. Y. Purification and characterization of a chloride-stimulated cellobiosidase from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2923–2932. doi: 10.1128/jb.170.7.2923-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Isolation and characterization of outer and inner membranes of Selenomonas ruminantium: lipid compositions. J Bacteriol. 1980 Feb;141(2):888–898. doi: 10.1128/jb.141.2.888-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Marsh P. D., Ellwood D. C. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J Bacteriol. 1984 Feb;157(2):560–567. doi: 10.1128/jb.157.2.560-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring S., Wiegel J., Mayer F. Subunit Composition and Glycosidic Activities of the Cellulase Complex from Clostridium thermocellum JW20. Appl Environ Microbiol. 1990 Dec;56(12):3798–3804. doi: 10.1128/aem.56.12.3798-3804.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Electron microscopic study of the methylcellulose-mediated detachment of cellulolytic rumen bacteria from cellulose fibers. Can J Microbiol. 1987 Mar;33(3):267–272. doi: 10.1139/m87-045. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linton J. D., Cripps R. E. The occurrence and identification of intracellular polyglucose storage granules in Methylococcus NCIB 11083 grown in chemostat culture on methane. Arch Microbiol. 1978 Apr 27;117(1):41–48. doi: 10.1007/BF00689349. [DOI] [PubMed] [Google Scholar]
- MacKenzie S. L. Gas chromatographic analysis of amino acids as the N-heptafluorobutyryl isobutyl esters. J Assoc Off Anal Chem. 1987 Jan-Feb;70(1):151–160. [PubMed] [Google Scholar]
- McGavin M. J., Forsberg C. W., Crosby B., Bell A. W., Dignard D., Thomas D. Y. Structure of the cel-3 gene from Fibrobacter succinogenes S85 and characteristics of the encoded gene product, endoglucanase 3. J Bacteriol. 1989 Oct;171(10):5587–5595. doi: 10.1128/jb.171.10.5587-5595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Catalytic and substrate-binding domains of endoglucanase 2 from Bacteroides succinogenes. J Bacteriol. 1989 Jun;171(6):3310–3315. doi: 10.1128/jb.171.6.3310-3315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Lam J., Forsberg C. W. Regulation and distribution of Fibrobacter succinogenes subsp. succinogenes S85 endoglucanases. Appl Environ Microbiol. 1990 May;56(5):1235–1244. doi: 10.1128/aem.56.5.1235-1244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. Annu Rev Biochem. 1986;55:287–315. doi: 10.1146/annurev.bi.55.070186.001443. [DOI] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Jespersen H., Sierks M. R., MacGregor E. A. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem J. 1989 Nov 15;264(1):309–311. doi: 10.1042/bj2640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zamze S. E., Ferguson M. A., Moxon E. R., Dwek R. A., Rademacher T. W. Identification of phosphorylated 3-deoxy-manno-octulosonic acid as a component of Haemophilus influenzae lipopolysaccharide. Biochem J. 1987 Jul 15;245(2):583–587. doi: 10.1042/bj2450583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn W. C. Glycogen, its chemistry and morphologic appearance in the electron microscope. I. A modified OsO 4 fixative which selectively contrasts glycogen. J Ultrastruct Res. 1973 Jan;42(1):29–50. doi: 10.1016/s0022-5320(73)80004-8. [DOI] [PubMed] [Google Scholar]