Abstract
Rhodospirillum rubrum and Rhodobacter sphaeroides were shown to be capable of photolithoautotrophic growth in the absence of the reductive pentose phosphate (Calvin) cycle. Ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) deletion strains were incapable of photolithoautotrophic growth using hydrogen as an electron donor but were able to grow in the absence of organic carbon using less reduced inorganic electron donors, i.e., thiosulfate or sulfide. Wild-type R. rubrum grown in the presence of thiosulfate contained RubisCO levels that were 50-fold lower compared with those in cells growth with hydrogen as an electron donor without substantially influencing rates of photolithoautotrophic growth. These results suggest there are two independent CO2 fixation pathways that support photolithoautotrophic growth in purple nonsulfur photosynthetic bacteria, indicating that these organisms have developed sophisticated control mechanisms to regulate the flow of carbon from CO2 through these separate pathways.
Full text
PDF
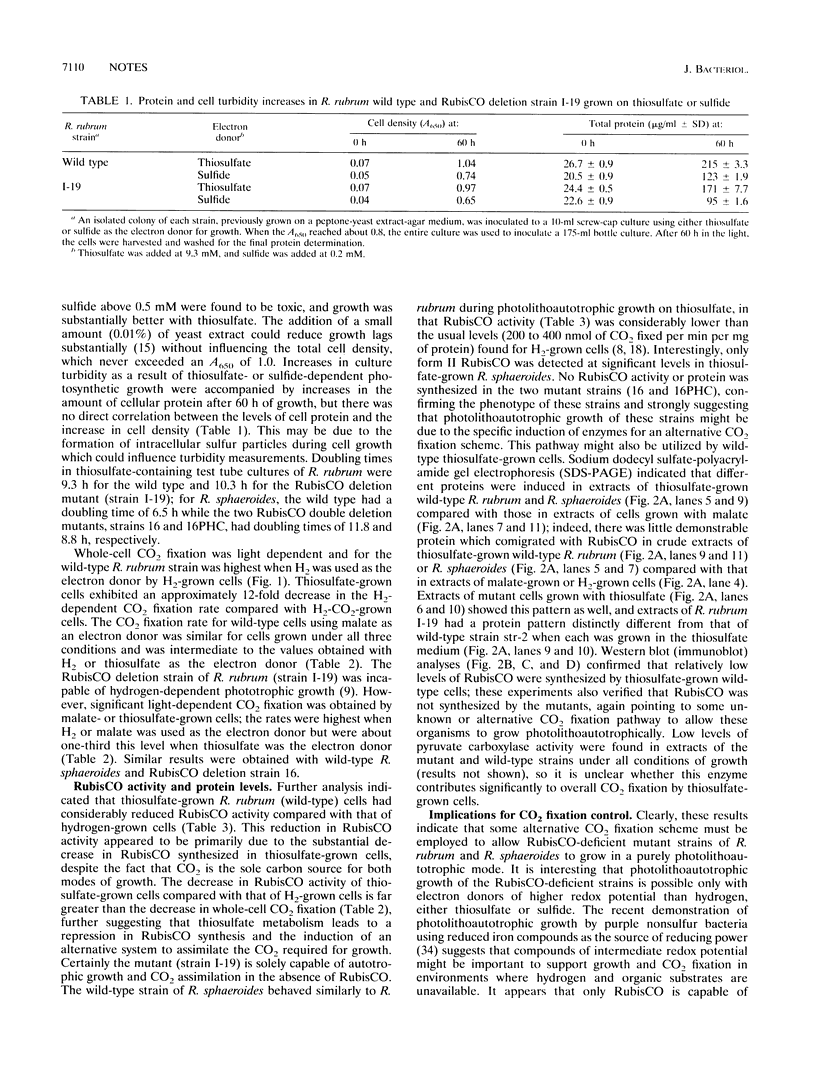
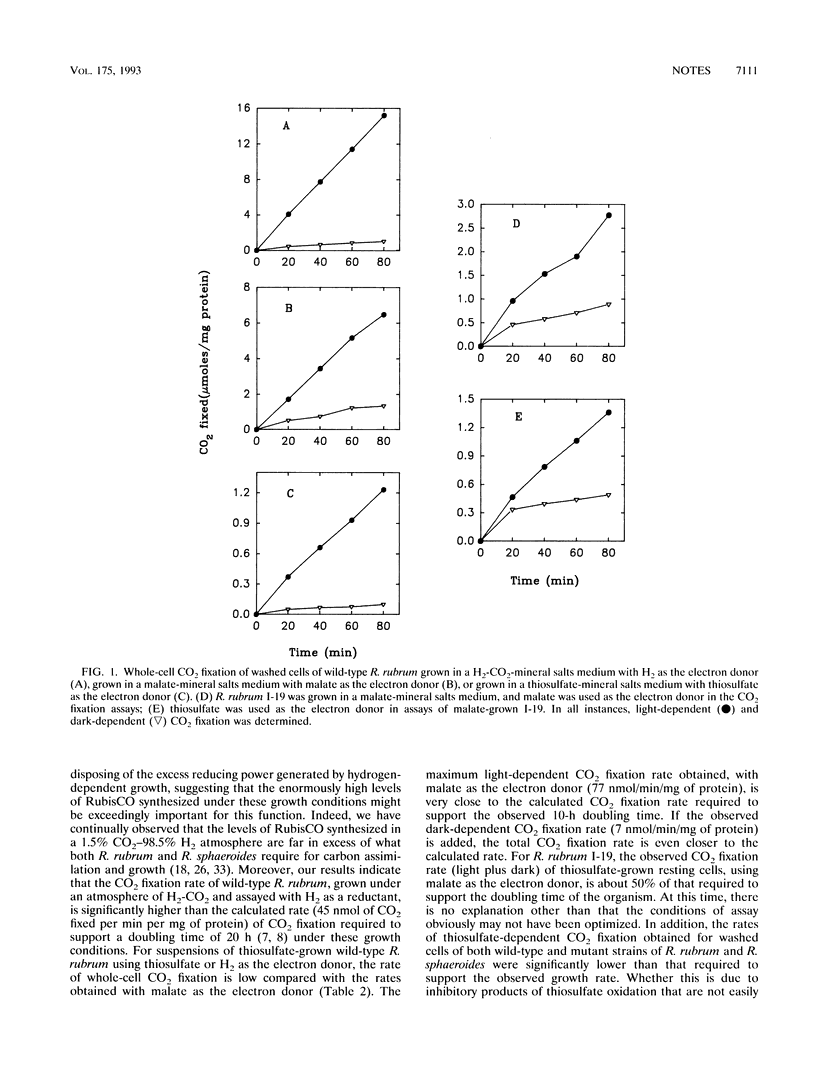
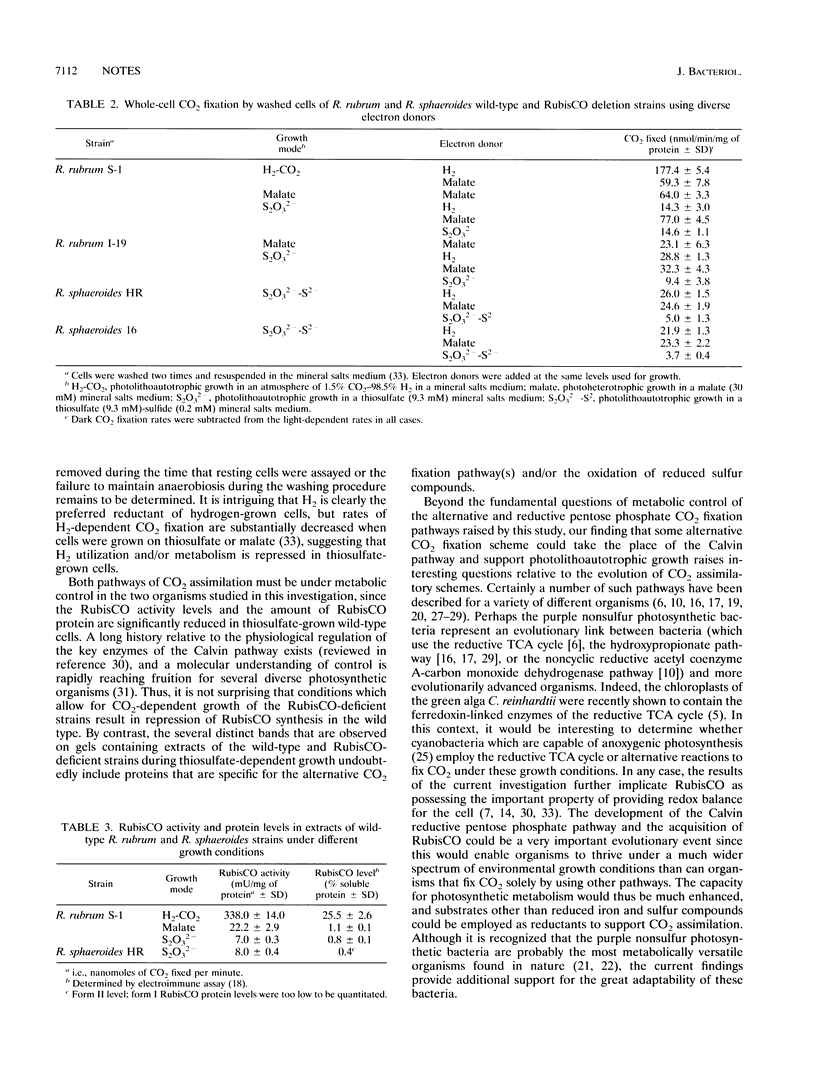
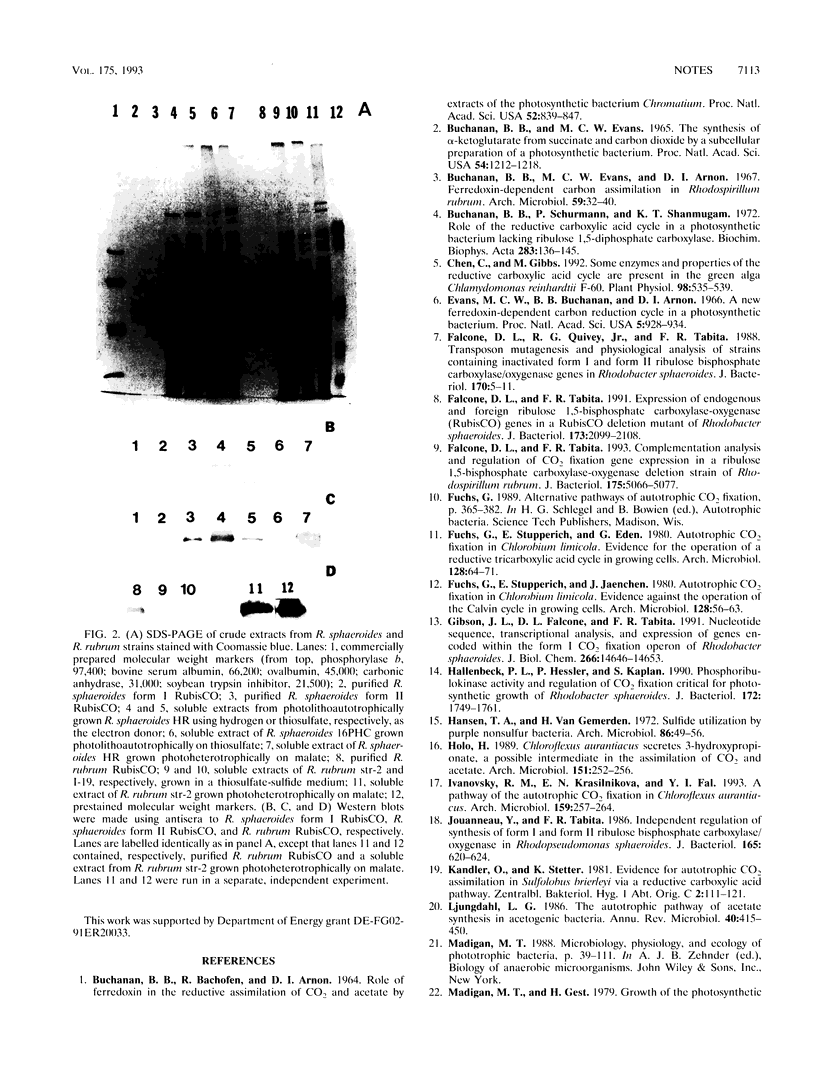

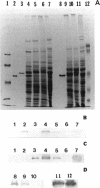
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C., Arnon D. I. Ferredoxin-dependent carbon assimilation in Rhodospirillum rubrum. Arch Mikrobiol. 1967;59(1):32–40. doi: 10.1007/BF00406314. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1212–1218. doi: 10.1073/pnas.54.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Shanmugam K. T. Role of the reductive carboxylic acid cycle in a photosynthetic bacterium lacking ribulose I,5-diphosphate carboxylase. Biochim Biophys Acta. 1972;283(1):136–145. doi: 10.1016/0005-2728(72)90105-3. [DOI] [PubMed] [Google Scholar]
- Chen C., Gibbs M. Some Enzymes and Properties of the Reductive Carboxylic Acid Cycle Are Present in the Green Alga Chlamydomonas reinhardtii F-60. Plant Physiol. 1992 Feb;98(2):535–539. doi: 10.1104/pp.98.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Quivey R. G., Jr, Tabita F. R. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):5–11. doi: 10.1128/jb.170.1.5-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Tabita F. R. Complementation analysis and regulation of CO2 fixation gene expression in a ribulose 1,5-bisphosphate carboxylase-oxygenase deletion strain of Rhodospirillum rubrum. J Bacteriol. 1993 Aug;175(16):5066–5077. doi: 10.1128/jb.175.16.5066-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Tabita F. R. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J Bacteriol. 1991 Mar;173(6):2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Falcone D. L., Tabita F. R. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Aug 5;266(22):14646–14653. [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1749–1761. doi: 10.1128/jb.172.4.1749-1761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. A., van Gemerden H. Sulfide utilization by purple nonsulfur bacteria. Arch Mikrobiol. 1972;86(1):49–56. doi: 10.1007/BF00412399. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Sarles L. S., Tabita F. R. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Bacteriol. 1983 Jan;153(1):458–464. doi: 10.1128/jb.153.1.458-464.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G., Eisenreich W., Bacher A., Fuchs G. 13C-NMR study of autotrophic CO2 fixation pathways in the sulfur-reducing Archaebacterium Thermoproteus neutrophilus and in the phototrophic Eubacterium Chloroflexus aurantiacus. Eur J Biochem. 1992 Apr 15;205(2):853–866. doi: 10.1111/j.1432-1033.1992.tb16850.x. [DOI] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Falcone D. L., Tabita F. R. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J Bacteriol. 1993 Jun;175(11):3372–3379. doi: 10.1128/jb.175.11.3372-3379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]