Abstract
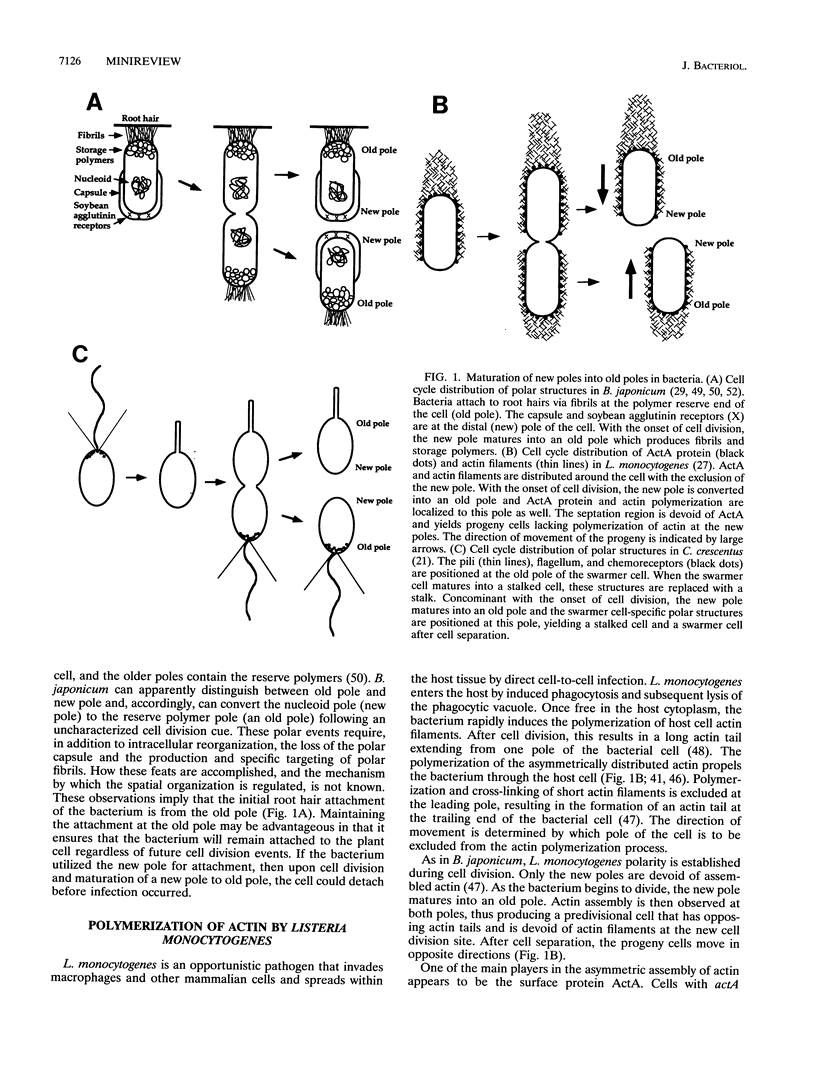
The recognition of polar bacterial organization is just emerging. The examples of polar localization given here are from a variety of bacterial species and concern a disparate array of cellular functions. A number of well-characterized instances of polar localization of bacterial proteins, including the chemoreceptor complex in both C. crescentus and E. coli, the maltose-binding protein in E. coli, the B. japonicum surface attachment proteins, and the actin tail of L. monocytogenes within a mammalian cell, involve proteins or protein complexes that facilitate bacterial interaction with the environment, either the extracellular milieux or that within a plant or mammalian host. The significance of this observation remains unclear. Polarity in bacteria poses many problems, including the necessity for a mechanism for asymmetrically distributing proteins as well as a mechanism by which polar localization is maintained. Large structures, such as a flagellum, are anchored at the pole by means of the basal body that traverses the peptidoglycan wall. But for proteins and small complexes, whether in the periplasm or the membrane, one must invoke a mechanism that prevents the diffusion of these proteins away from the cell pole. Perhaps the periplasmic proteins are retained at the pole by the presence of the periseptal annulus (35). The constraining features for membrane components are not known. For large aggregates, such as the clusters of MCP, CheA, and CheW complexes, perhaps the size of the aggregate alone prevents displacement. In most cases of cellular asymmetry, bacteria are able to discriminate between the new pole and the old pole and to utilize this information for localization specificity. The maturation of new pole to old pole appears to be a common theme as well. Given numerous examples reported thus far, we propose that bacterial polarity displays specific rules and is a more general phenomenon than has been previously recognized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Shi W. Galvanotaxis in bacteria. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):23–25. doi: 10.1101/sqb.1988.053.01.006. [DOI] [PubMed] [Google Scholar]
- Alley M. R., Maddock J. R., Shapiro L. Polar localization of a bacterial chemoreceptor. Genes Dev. 1992 May;6(5):825–836. doi: 10.1101/gad.6.5.825. [DOI] [PubMed] [Google Scholar]
- Alley M. R., Maddock J. R., Shapiro L. Requirement of the carboxyl terminus of a bacterial chemoreceptor for its targeted proteolysis. Science. 1993 Mar 19;259(5102):1754–1757. doi: 10.1126/science.8456303. [DOI] [PubMed] [Google Scholar]
- Angert E. R., Clements K. D., Pace N. R. The largest bacterium. Nature. 1993 Mar 18;362(6417):239–241. doi: 10.1038/362239a0. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E. Bacteriophage SP50 as a marker for cell wall growth in Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1195–1206. doi: 10.1128/jb.125.3.1195-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizono T., Umeda A., Amako K. Distribution of capsular materials on the cell wall surface of strain Smith diffuse of Staphylococcus aureus. J Bacteriol. 1991 Jul;173(14):4333–4340. doi: 10.1128/jb.173.14.4333-4340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A. K., Shantharam S., Ratnam S. Ultrastructure of Rhizobium japonicum in relation to its attachment to root hairs. J Bacteriol. 1978 Mar;133(3):1393–1400. doi: 10.1128/jb.133.3.1393-1400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Doanachie W. D. Growth of the Escherichia coli cell surface. J Bacteriol. 1977 Mar;129(3):1524–1536. doi: 10.1128/jb.129.3.1524-1536.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E. F., Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991 Nov 14;354(6349):161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Immunofluorescent polar tips of Rhizobium japonicum: possible site of attachment or lectin binding. J Bacteriol. 1976 Mar;125(3):1188–1194. doi: 10.1128/jb.125.3.1188-1194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Staehelin A. L. Ultrastructural localization of the maltose-binding protein within the cell envelope of Escherichia coli. Arch Microbiol. 1981 May;129(3):240–246. doi: 10.1007/BF00425258. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Clements K. D., Bullivant S. An unusual symbiont from the gut of surgeonfishes may be the largest known prokaryote. J Bacteriol. 1991 Sep;173(17):5359–5362. doi: 10.1128/jb.173.17.5359-5362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I., Kolb V., Boos W. Pole cap formation in Escherichia coli following induction of the maltose-binding protein. Arch Microbiol. 1978 Aug 1;118(2):207–218. doi: 10.1007/BF00415731. [DOI] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Wetzel B. K., Heppel L. A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a "minicell" strain of Escherichia coli. J Bacteriol. 1970 Oct;104(1):543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E., García E., Ascaso C., Méndez E., López R., García J. L. Subcellular localization of the major pneumococcal autolysin: a peculiar mechanism of secretion in Escherichia coli. J Biol Chem. 1989 Jan 15;264(2):1238–1244. [PubMed] [Google Scholar]
- Gegner J. A., Graham D. R., Roth A. F., Dahlquist F. W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992 Sep 18;70(6):975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Alley M. R., Shapiro L. Positional information during Caulobacter cell differentiation. Curr Opin Genet Dev. 1991 Oct;1(3):324–329. doi: 10.1016/s0959-437x(05)80295-3. [DOI] [PubMed] [Google Scholar]
- Goldberg M. B., Bârzu O., Parsot C., Sansonetti P. J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993 Apr;175(8):2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. C., Schindler M., Wang J. L. Carbohydrate binding activities of Bradyrhizobium japonicum. II. Isolation and characterization of a galactose-specific lectin. J Cell Biol. 1990 Oct;111(4):1639–1643. doi: 10.1083/jcb.111.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. C., Wang J. L., Schindler M. Carbohydrate binding activities of Bradyrhizobium japonicum. I. Saccharide-specific inhibition of homotypic and heterotypic adhesion. J Cell Biol. 1990 Oct;111(4):1631–1638. doi: 10.1083/jcb.111.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenel E. D., Newton A. Localization of surface structures during procaryotic differentiation: role of cell division in Caulobacter crescentus. Differentiation. 1982;21(2):71–78. doi: 10.1111/j.1432-0436.1982.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kocks C., Hellio R., Gounon P., Ohayon H., Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993 Jul;105(Pt 3):699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- Loh J. T., Ho S. C., de Feijter A. W., Wang J. L., Schindler M. Carbohydrate binding activities of Bradyrhizobium japonicum: unipolar localization of the lectin BJ38 on the bacterial cell surface. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3033–3037. doi: 10.1073/pnas.90.7.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukat G. S., Stock J. B. Response regulation in bacterial chemotaxis. J Cell Biochem. 1993 Jan;51(1):41–46. doi: 10.1002/jcb.240510109. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. FtsZ ring in bacterial cytokinesis. Mol Microbiol. 1993 Aug;9(3):403–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Cook W. R., Weigand R., Rothfield L. I. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis of the gram-negative bacterial division septum. J Bacteriol. 1987 Sep;169(9):3945–3951. doi: 10.1128/jb.169.9.3945-3951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Thompson L., Thompson J., Ingram J. M. Distribution of alkaline phosphatase within the periplasmic space of gram-negative bacteria. J Bacteriol. 1972 Sep;111(3):827–832. doi: 10.1128/jb.111.3.827-832.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae T. H., McCurdy D. Evidence for motility-related fimbriae in the gliding microorganism Myxococcus xanthus. Can J Microbiol. 1976 Oct;22(10):1589–1593. doi: 10.1139/m76-234. [DOI] [PubMed] [Google Scholar]
- MacaAlister T. J., Irvin R. T., Costerton J. W. Cell surface-localized alkaline phosphatase of Escherichia coli as visualized by reaction product deposition and ferritin-labeled antibodies. J Bacteriol. 1977 Apr;130(1):318–328. doi: 10.1128/jb.130.1.318-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock J. R., Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993 Mar 19;259(5102):1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Cumsky M. G., Zusman D. R. Localization of myxobacterial hemagglutinin in the periplasmic space and on the cell surface of Myxococcus xanthus during developmental aggregation. J Biol Chem. 1981 Dec 10;256(23):12589–12595. [PubMed] [Google Scholar]
- Parkinson J. S. Signal transduction schemes of bacteria. Cell. 1993 Jun 4;73(5):857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W., Southwick F. S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992 Sep;60(9):3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L. Initiation of plant root-microbe interactions. Annu Rev Microbiol. 1979;33:355–376. doi: 10.1146/annurev.mi.33.100179.002035. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., Hebert R. R. Adsorption of rhizobia to cereal roots. Biochem Biophys Res Commun. 1978 Oct 16;84(3):736–742. doi: 10.1016/0006-291x(78)90766-0. [DOI] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992 Jul;118(1):71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Localization and partial characterization of soybean lectin-binding polysaccharide of Rhizobium japonicum. J Bacteriol. 1981 Feb;145(2):1063–1074. doi: 10.1128/jb.145.2.1063-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- Vesper S. J., Bauer W. D. Role of Pili (Fimbriae) in Attachment of Bradyrhizobium japonicum to Soybean Roots. Appl Environ Microbiol. 1986 Jul;52(1):134–141. doi: 10.1128/aem.52.1.134-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993 Aug;9(3):435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Wanner G., Formanek H., Galli D., Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151(6):491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- Xia T., Song J., Zhao G., Aldrich H., Jensen R. A. The aroQ-encoded monofunctional chorismate mutase (CM-F) protein is a periplasmic enzyme in Erwinia herbicola. J Bacteriol. 1993 Aug;175(15):4729–4737. doi: 10.1128/jb.175.15.4729-4737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Xia T., Aldrich H., Jensen R. A. Cyclohexadienyl dehydratase from Pseudomonas aeruginosa is a periplasmic protein. J Gen Microbiol. 1993 Apr;139(4):807–813. doi: 10.1099/00221287-139-4-807. [DOI] [PubMed] [Google Scholar]
- Zoutman D. E., Hulbert W. C., Pasloske B. L., Joffe A. M., Volpel K., Trebilcock M. K., Paranchych W. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphologic study. Scanning Microsc. 1991 Mar;5(1):109–126. [PubMed] [Google Scholar]


