Abstract
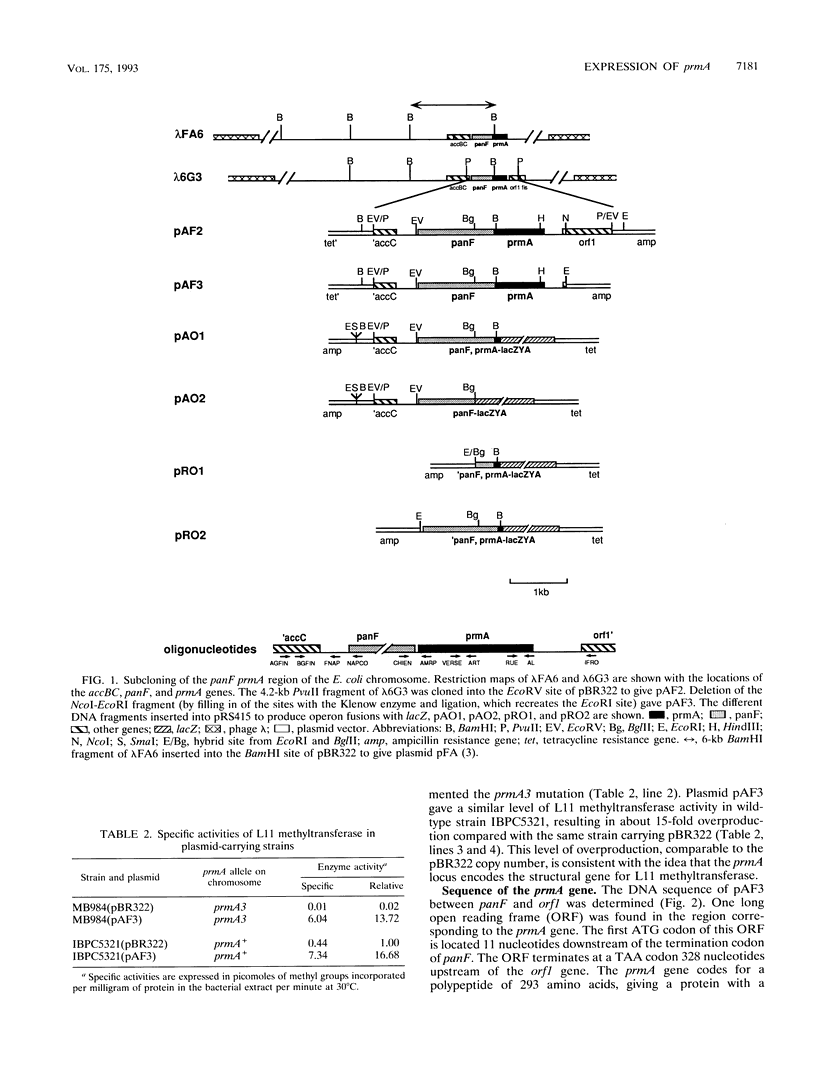
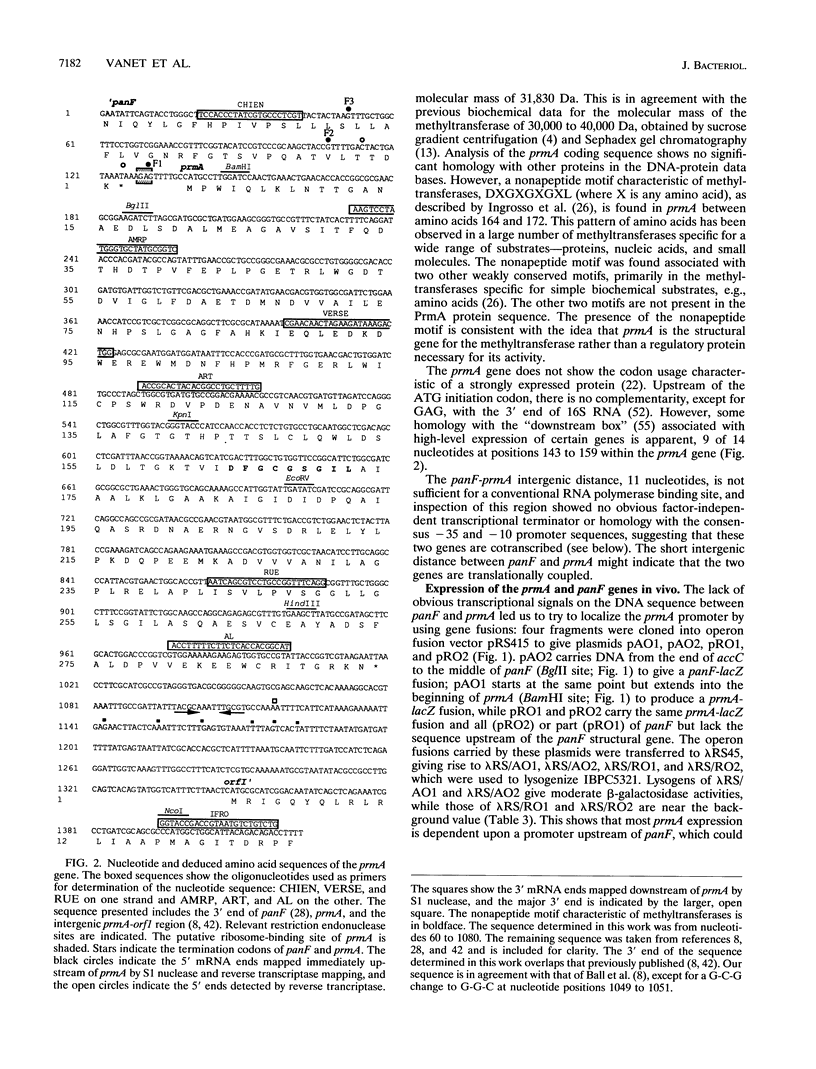
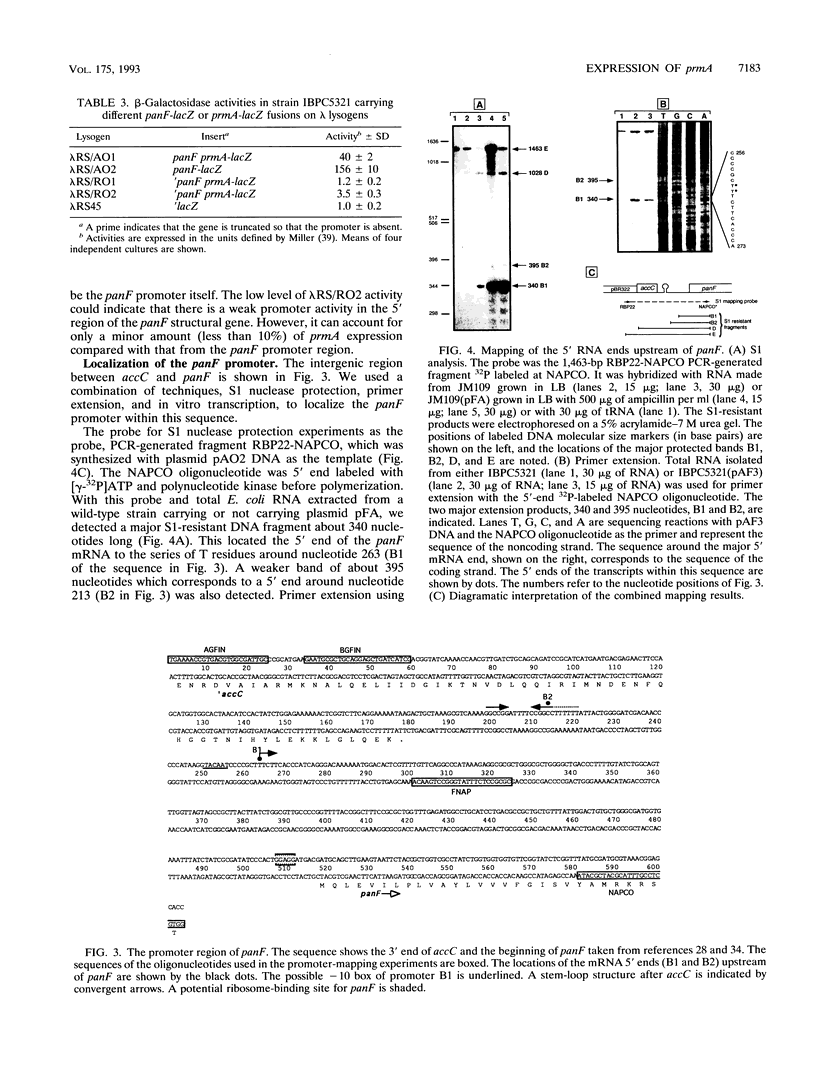
Genetic complementation and enzyme assays have shown that the DNA region between panF, which encodes pantothenate permease, and orf1, the first gene of the fis operon, encodes prmA, the genetic determinant for the ribosomal protein L11 methyltransferase. Sequencing of this region identified one long open reading frame that encodes a protein of 31,830 Da and corresponds to the prmA gene. We found, both in vivo and in vitro, that prmA is expressed from promoters located upstream of panF and thus that the panF and prmA genes constitute a bifunctional operon. We located the major 3' end of prmA transcripts 90 nucleotides downstream of the stop codon of prmA in the DNA region upstream of the fis operon, a region implicated in the control of the expression of the fis operon. Although no promoter activity was detected immediately upstream of prmA, S1 mapping detected 5' ends of mRNA in this region, implying that some mRNA processing occurs within the bicistronic panF-prmA mRNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix J. H. A rapid procedure for cloning genes from lambda libraries by complementation of E. coli defective mutants: application to the fabE region of the E. coli chromosome. DNA. 1989 Dec;8(10):779–789. doi: 10.1089/dna.1989.8.779. [DOI] [PubMed] [Google Scholar]
- Alix J. H., Hayes D., Lontie J. F., Colson C., Glatigny A., Lederer F. Methylated amino acids in ribosomal proteins from Escherichia coli treated with ethionine and from a mutant lacking methylation of protein L11. Biochimie. 1979;61(5-6):671–679. doi: 10.1016/s0300-9084(79)80165-0. [DOI] [PubMed] [Google Scholar]
- Alix J. H., Hayes D. Properties of ribosomes and RNA synthesized by Escherichia coli grown in the presence of ethionine. 3. Methylated proteins in 50 S ribosomes of E. coli EA2. J Mol Biol. 1974 Jun 15;86(1):139–159. doi: 10.1016/s0022-2836(74)80013-6. [DOI] [PubMed] [Google Scholar]
- Alix J. H. Post-translational methylations of ribosomal proteins. Adv Exp Med Biol. 1988;231:371–385. doi: 10.1007/978-1-4684-9042-8_30. [DOI] [PubMed] [Google Scholar]
- Ambulos N. P., Jr, Rogers E. J., Alexieva Z., Lovett P. S. Induction of cat-86 by chloramphenicol and amino acid starvation in relaxed mutants of Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5642–5646. doi: 10.1128/jb.170.12.5642-5646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong I. L., Tate W. P. Requirement for the Escherichia coli ribosomal protein L11 in peptide chain termination. J Mol Biol. 1978 Apr 5;120(2):155–166. doi: 10.1016/0022-2836(78)90062-1. [DOI] [PubMed] [Google Scholar]
- Ball C. A., Osuna R., Ferguson K. C., Johnson R. C. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992 Dec;174(24):8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. M., Zahid N. L16, a bifunctional ribosomal protein and the enhancing effect of L6 and L11. Eur J Biochem. 1986 Mar 3;155(2):273–277. doi: 10.1111/j.1432-1033.1986.tb09486.x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Cohen L. B., Navickas I. J., Chang C. N. Purification and properties of a ribosomal protein methylase from Eschericha coli Q13. Biochemistry. 1975 Nov 4;14(22):4994–4998. doi: 10.1021/bi00693a032. [DOI] [PubMed] [Google Scholar]
- Colson C. Genetics of ribosomal protein methylation in Escherichia coli. I. A mutant deficient in methylation of protein L11. Mol Gen Genet. 1977 Jul 20;154(2):167–173. doi: 10.1007/BF00330832. [DOI] [PubMed] [Google Scholar]
- Colson C., Lhoest J., Urlings C. Genetics of ribosomal protein methylation in Escherichia coli. III. Map position of two genes, prmA and prmB, governing methylation of proteins L11 and L3. Mol Gen Genet. 1979 Feb 1;169(3):245–250. doi: 10.1007/BF00382270. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Mutational alterations in 50 proteins of the Escherichia coli ribosome. Mol Gen Genet. 1978 Sep 20;165(1):73–78. doi: 10.1007/BF00270378. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. The ribosomal components responsible for kasugamycin dependence, and its suppression, in a mutant of Escherichia coli. Mol Gen Genet. 1980 Jan;177(2):271–276. doi: 10.1007/BF00267438. [DOI] [PubMed] [Google Scholar]
- Dognin M. J., Wittmann-Liebold B. Identification of methylated amino acids during sequence analysis. Application to the Escherichia coli ribosomal protein L11. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1697–1705. doi: 10.1515/bchm2.1980.361.2.1697. [DOI] [PubMed] [Google Scholar]
- Dognin M. J., Wittmann-Liebold B. Purification and primary structure determination of the N-terminal blocked protein, L11, from Escherichia coli ribosomes. Eur J Biochem. 1980 Nov;112(1):131–151. doi: 10.1111/j.1432-1033.1980.tb04995.x. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Douthwaite S. R., Liljas A., Garrett R. A. Characterization of the binding sites of protein L11 and the L10.(L12)4 pentameric complex in the GTPase domain of 23 S ribosomal RNA from Escherichia coli. J Mol Biol. 1990 May 20;213(2):275–288. doi: 10.1016/S0022-2836(05)80190-1. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Götz F., Fleischer C., Pon C. L., Gualerzi C. O. Subunit association defects in Escherichia coli ribosome mutants lacking proteins S20 and L11. Eur J Biochem. 1989 Jul 15;183(1):19–24. doi: 10.1111/j.1432-1033.1989.tb14890.x. [DOI] [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Hellmuth K., Rex G., Surin B., Zinck R., McCarthy J. E. Translational coupling varying in efficiency between different pairs of genes in the central region of the atp operon of Escherichia coli. Mol Microbiol. 1991 Apr;5(4):813–824. doi: 10.1111/j.1365-2958.1991.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Hitz H., Schafer D., Wittmann-Liebold B. Primary structure of ribosomal protein S6 from the wild type and a mutant of Escherichia coli. FEBS Lett. 1975 Aug 15;56(2):259–262. doi: 10.1016/0014-5793(75)81105-7. [DOI] [PubMed] [Google Scholar]
- Ingrosso D., Fowler A. V., Bleibaum J., Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [PubMed] [Google Scholar]
- Isono K., Krauss J., Hirota Y. Isolation and characterization of temperature-sensitive mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1976 Dec 22;149(3):297–302. doi: 10.1007/BF00268531. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Alix J. H. Cloning, sequence, and expression of the pantothenate permease (panF) gene of Escherichia coli. J Bacteriol. 1990 Jul;172(7):3842–3848. doi: 10.1128/jb.172.7.3842-3848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W. K., Icho T., Isono S., Kitakawa M., Isono K. Characterization of the gene rimK responsible for the addition of glutamic acid residues to the C-terminus of ribosomal protein S6 in Escherichia coli K12. Mol Gen Genet. 1989 Jun;217(2-3):281–288. doi: 10.1007/BF02464894. [DOI] [PubMed] [Google Scholar]
- Kazemie M. The importance of Escherichia coli ribosomal proteins L1, L11 and L16 for the association of ribosomal subunits and the formation of the 70-S initiation complex. Eur J Biochem. 1975 Oct 15;58(2):501–510. doi: 10.1111/j.1432-1033.1975.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Koch C., Vandekerckhove J., Kahmann R. Escherichia coli host factor for site-specific DNA inversion: cloning and characterization of the fis gene. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4237–4241. doi: 10.1073/pnas.85.12.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kondo H., Shiratsuchi K., Yoshimoto T., Masuda T., Kitazono A., Tsuru D., Anai M., Sekiguchi M., Tanabe T. Acetyl-CoA carboxylase from Escherichia coli: gene organization and nucleotide sequence of the biotin carboxylase subunit. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9730–9733. doi: 10.1073/pnas.88.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer F., Alix J. H., Hayes D. N-Trimethylalanine, a novel blocking group, found in E. coli ribosomal protein L11. Biochem Biophys Res Commun. 1977 Jul 25;77(2):470–480. doi: 10.1016/s0006-291x(77)80004-1. [DOI] [PubMed] [Google Scholar]
- Lesage P., Chiaruttini C., Graffe M., Dondon J., Milet M., Springer M. Messenger RNA secondary structure and translational coupling in the Escherichia coli operon encoding translation initiation factor IF3 and the ribosomal proteins, L35 and L20. J Mol Biol. 1992 Nov 20;228(2):366–386. doi: 10.1016/0022-2836(92)90827-7. [DOI] [PubMed] [Google Scholar]
- Lhoest J., Colson C. Cold-sensitive ribosome assembly in an Escherichia coli mutant lacking a single methyl group in ribosomal protein L3. Eur J Biochem. 1981 Dec;121(1):33–37. doi: 10.1111/j.1432-1033.1981.tb06425.x. [DOI] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Jan 15;267(2):855–863. [PubMed] [Google Scholar]
- Naaktgeboren N., Schrier P., Möller W., Voorma H. O. The involvement of protein L11 in the joining of the 30-S initiation complex to the 50-S subunit. Eur J Biochem. 1976 Feb 2;62(1):117–123. doi: 10.1111/j.1432-1033.1976.tb10104.x. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann O., Koch C., Kahmann R. The E.coli fis promoter is subject to stringent control and autoregulation. EMBO J. 1992 Mar;11(3):1075–1083. doi: 10.1002/j.1460-2075.1992.tb05146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K. A relaxed (rel) mutant of Streptomyces coelicolor A3(2) with a missing ribosomal protein lacks the ability to accumulate ppGpp, A-factor and prodigiosin. J Gen Microbiol. 1990 Dec;136(12):2405–2412. doi: 10.1099/00221287-136-12-2405. [DOI] [PubMed] [Google Scholar]
- Ochi K. Streptomyces relC mutants with an altered ribosomal protein ST-L11 and genetic analysis of a Streptomyces griseus relC mutant. J Bacteriol. 1990 Jul;172(7):4008–4016. doi: 10.1128/jb.172.7.4008-4016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Watson R. J., Friesen J. D. A relaxed mutant with an altered ribosomal protein L11. Mol Gen Genet. 1976 Feb 27;144(1):111–114. doi: 10.1007/BF00277313. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Dondon J., Nakamura Y., Grunberg-Manago M. Effect of NusA protein on expression of the nusA,infB operon in E. coli. Nucleic Acids Res. 1985 May 10;13(9):3371–3388. doi: 10.1093/nar/13.9.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Organization of the Escherichia coli chromosome around the genes for translation initiation factor IF2 (infB) and a transcription termination factor (nusA). J Mol Biol. 1983 Jun 25;167(2):227–243. doi: 10.1016/s0022-2836(83)80333-7. [DOI] [PubMed] [Google Scholar]
- Ryan P. C., Draper D. E. Detection of a key tertiary interaction in the highly conserved GTPase center of large subunit ribosomal RNA. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6308–6312. doi: 10.1073/pnas.88.14.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Seto D. An improved method for sequencing double stranded plasmid DNA from minipreps using DMSO and modified template preparation. Nucleic Acids Res. 1990 Oct 11;18(19):5905–5906. doi: 10.1093/nar/18.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Sprengart M. L., Fatscher H. P., Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16srRNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990 Apr 11;18(7):1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Cundliffe E., Stöffler-Meilicke M., Dabbs E. R. Mutants of Escherichia coli lacking ribosomal protein L11. J Biol Chem. 1980 Nov 10;255(21):10517–10522. [PubMed] [Google Scholar]
- Stöffler G., Hasenbank R., Dabbs E. R. Expression of the L11-L1 operon in mutants of Escherichia coli lacking the ribosomal proteins L1 or L11. Mol Gen Genet. 1981;181(2):164–168. doi: 10.1007/BF00268422. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Dognin M. J., Noah M., Stöffler-Meilicke M., Stöffler G. The NH2-terminal domain of Escherichia coli ribosomal protein L11. Its three-dimensional location and its role in the binding of release factors 1 and 2. J Biol Chem. 1984 Jun 10;259(11):7317–7324. [PubMed] [Google Scholar]
- Tate W. P., Schulze H., Nierhaus K. H. The Escherichia coli ribosomal protein L11 suppresses release factor 2 but promotes the release factor 1 activities in peptide chain termination. J Biol Chem. 1983 Nov 10;258(21):12816–12820. [PubMed] [Google Scholar]
- Thompson J., Cundliffe E., Stark M. Binding of thiostrepton to a complex of 23-S rRNA with ribosomal protein L11. Eur J Biochem. 1979 Jul;98(1):261–265. doi: 10.1111/j.1432-1033.1979.tb13184.x. [DOI] [PubMed] [Google Scholar]
- Uzan M., Favre R., Brody E. A nuclease that cuts specifically in the ribosome binding site of some T4 mRNAs. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8895–8899. doi: 10.1073/pnas.85.23.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallari D. S., Rock C. O. Pantothenate transport in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1156–1161. doi: 10.1128/jb.162.3.1156-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]






